|
|
 |
| ............................................................. |
|
|
| ........................................................ |
| From
the Editor |

|
Editorial
A. Abyad (Chief Editor) |
|
|
|
|
........................................................ |
Original
Contribution / Clinical Investigation
|





|
<-- Jordan, USA -->
Herpetic
Eye Disease and Glaucoma Related Diagnosis
[pdf version]
C. Dan Earley, Amal M Althawabi,
Paul R Cotran, Sarkis H Soukiasian
<-- Turkey, Lebanon, Australia -->
Cholelithiasis
may also be a consequence of metabolic syndrome
[pdf
version]
Mehmet Rami Helvaci, Mursel Davarci,
Orhan Veli Ozkan, Ersan Semerci, Abdulrazak
Abyad, Lesley Pocock
<-- Iran -->
SUMO1 pseudogene
3 (SUMO1P3) expression in human gastric cancer
and its clinical significance
[pdf version]
Hamid Reza Baradaran-Ghahfarokhi, Habib Malekpour,
Ehsan Nazemalhosseini Mojarad,
Hamid Asadzadeh Aghdaei, Majid Asadi-Samani,
Azar Baradaran
<-- Iran -->
Decoy Cell
Viruria in Kidney Transplant Patients. Does
it correlate with Renal Function?
[pdf version]
Akram Abedi, Mojgan Mortazavi,
Omid Mirmosayyeb, Shahram Taheri,
Nooshin Afsharmoghadam,
Majid Asadi-Samani, Shahram Sajadieh,
Azar Baradaran
<-- Iran, Austria -->
To determine
how frequently pregnant asthmatics are sensitive
to food and inhalation allergens
[pdf version]
Nasrin Fazel, Michael Kundi,
Erika Jensen-Jarolim,
Isabella Maria Pali-Schöll,
Asghar Kazemzadeh, Mojtaba Fattahi Abdizadeh,
Habibollah Esmaily,
Roya Akbarzadeh, Raheleh Ahmadi
|
........................................................
Special Education Feature
........................................................
International Health
Affairs
|
Chief
Editor -
Abdulrazak
Abyad
MD, MPH, MBA, AGSF, AFCHSE
.........................................................
Editorial
Office -
Abyad Medical Center & Middle East Longevity
Institute
Azmi Street, Abdo Center,
PO BOX 618
Tripoli, Lebanon
Phone: (961) 6-443684
Fax: (961) 6-443685
Email:
aabyad@cyberia.net.lb
.........................................................
Publisher
-
Lesley
Pocock
medi+WORLD International
11 Colston Avenue,
Sherbrooke 3789
AUSTRALIA
Phone: +61 (3) 9005 9847
Fax: +61 (3) 9012 5857
Email:
lesleypocock@mediworld.com.au
.........................................................
Editorial
Enquiries -
abyad@cyberia.net.lb
.........................................................
Advertising
Enquiries -
lesleypocock@mediworld.com.au
.........................................................
While all
efforts have been made to ensure the accuracy
of the information in this journal, opinions
expressed are those of the authors and do not
necessarily reflect the views of The Publishers,
Editor or the Editorial Board. The publishers,
Editor and Editorial Board cannot be held responsible
for errors or any consequences arising from
the use of information contained in this journal;
or the views and opinions expressed. Publication
of any advertisements does not constitute any
endorsement by the Publishers and Editors of
the product advertised.
The contents
of this journal are copyright. Apart from any
fair dealing for purposes of private study,
research, criticism or review, as permitted
under the Australian Copyright Act, no part
of this program may be reproduced without the
permission of the publisher.
|
|
|
| July 2017 - Volume
15, Issue 5 |
|
|
Decoy Cell Viruria in
Kidney Transplant Patients. Does it correlate
with Renal Function?
Akram
Abedi (1)
Mojgan Mortazavi (2)
Omid Mirmosayyeb (3,4)
Shahram Taheri (2)
Nooshin Afsharmoghadam
(1)
Majid Asadi-Samani
(5)
Shahram Sajadieh
(2)
Azar Baradaran
(1)
(1) Department of Pathology, School of Medicine,
Isfahan University of Medical Sciences, Isfahan,
Iran
(2) Department of Nephrology, Isfahan Kidney
Diseases Research Center, Isfahan University
of Medical Sciences, Isfahan, Iran
(3) Department of Neurology, Isfahan Neurosciences
Research Center, Al Zahra Hospital, Isfahan
University of Medical Sciences, Isfahan, Iran
(4) Student Research Committee, Isfahan University
of Medical Sciences, Isfahan, Iran
(5) Student Research Committee, Shahrekord University
of Medical Sciences, Shahrekord, Iran
Correspondence:
Professor Azar Baradaran
Department of Pathology,
School of Medicine,
Isfahan University of Medical Sciences,
Isfahan,
Iran
Email: azarbaradaran@med.mui.ac.ir
|
Abstract
Objective:
BK virus (BKV) infection after kidney
transplantation has been a topic of great
interest in the recent decade. Prospective
screening studies have revealed that BKVN
is principally an early complication of
renal transplantation occurring within
the first post-transplant year in most
cases. The aim of the present study was
to observe the incidence of decoy cell
viruria in renal transplant recipients.
Furthermore, correlation of decoy cell
viruria with graft function was assessed.
Methods: This analytic cross-sectional
study was conducted in the Transplant
Center of Alzahra Hospital, Isfahan, Iran
between Jun 2014 and June 2015. Clinical
screening for polyomavirus infection was
done by means of urine cytological evaluation
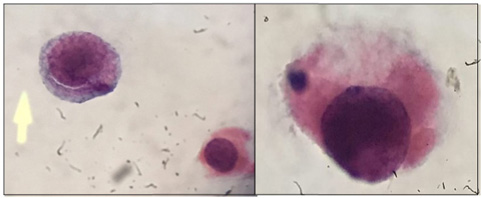
for decoy cells. Urine samples were analyzed
in three steps including 2-4 months after
transplantation, three and six months
later.
Results: Thirty-three patients
(22 male and 11 female) received kidney
transplant from living donors. The average
of patients' age was 41.9±12.83
(range: 20-63 years). Peritoneal and hemodialysis
were used for 15.6% and 84.4% of recipients.
The occurrence of decoy cell viruria at
the time of enrollment, 3 and 6 months
later was found in 18.2%, 10.7% and zero,
respectively.
Conclusion: As urine cytology is
easy to perform and of low cost, it is
a useful tool for the investigation of
active polyoma virus infection. Moreover,
the findings advocate that the presence
of decoy cells along with high creatinine
is a better indicator of the virus presence.
Key words:
BK Virus, Decoy Cell Viruria, Renal Transplantation,
Renal Function
|
BK virus (BKV) infection after kidney transplantation
has been a topic of great interest in the recent
decade. Human polyoma viruses are the members
of the papova virus family which have a double
strand DNA genome. The most identified species
of this kind are BK-virus, JC-virus (JCV) and
Simian-virus. BKV was first isolated from the
urine of a renal transplant recipient with ureteric
stenosis in 1971, but until 20 years later BKV
was not recognized as a reason of interstitial
nephritis and allograft failure in renal transplant
patients. The preliminary infection may occur
through fecal-oral transmission, respiratory tract
and over the placenta. Also, they can be transmitted
through organ transplantation. The vast majority
of polyomavirus associated nephropathy (PVN) is
triggered by the BKV, and the JCV is responsible
for less than 3% of cases. (1)
BKV nephropathy (BKVN) which is involving 1-7%
of renal transplant recipients, presented as a
slow increase of serum creatinine. Prospective
screening studies have revealed that BKVN is principally
an early complication of renal transplantation
occurring within the first post-transplant year
in most cases. (2) Although the pathological view
of tubulointerstitial nephritis can mimic rejection,
the treatments for these two conditions are dissimilar:
While dose reduction of immunosuppressant is the
treatment of tubulointerstitial nephritis, treatment
of rejection is by increase in immunosuppressant
dose. (3)
As BKVN has restricted treatment options, the
goal of screening is to facilitate primary diagnosis
of patients when viruric or viremic, and to interfere
before the development of overt nephropathy. After
BK recurrence, the virus is first detectable in
the urine, however, viremia develops after several
weeks. Despite guidelines recommending quantitative
polymerase chain reaction (PCR) for screening,
urinary decoy cell detection is a potentially
cost-effective alternative. (4) The aim of the
present study was to observe the incidence of
decoy cell viruria in renal transplant recipients.
Furthermore, correlation of decoy cell viruria
with graft function was assessed.
Recruiting patients
This analytic cross-sectional study was conducted
in the Transplant Center of Alzahra hospital,
Isfahan, Iran between Jun 2014 and June 2015.
Ethical approval was attained from the local
research ethics committee in school of medicine,
Isfahan University of Isfahan before enrollment.
(Approval code: IR.MUI.REC.1393.3030367, research
project code: 393367) Informed written consent
was obtained from all cases before recruiting
in the study. Consecutive kidney transplant
recipients from living donors who were older
than 18 years were included. The inclusion criteria
were to pass 1-4 months from transplantation.
Patients who had a positive history of acute
renal rejection or urothelial cancers were excluded.
Also, patients were excluded from the study
if they were unable to continue due to any causes.
In all patients a comprehensive questionnaire
including recipient demographic features, past
drug history, concomitant diseases, type and
duration of dialysis and time after transplant
were recorded.
Laboratory tests
Clinical screening for polyomavirus infection
was done by means of urine cytological evaluation
for decoy cells. Urine samples were analyzed
in three steps including 2-4 months after transplantation,
three and six months later. Early in the morning
the patient voided the urine collected in the
bladder overnight; the next fresh urine sample
was referred to cytology laboratory within 15
minutes of micturition; 0.5-1 mL of urine was
processed by liquid based cytology. Slides were
immediately fixed in 95% alcohol for Papanicolaou
staining. Time interval between the day of transplantation
and first appearance of decoy cells in the urine
and period of decoy cell persistence in the
urine were assessed. Also, the number of decoy
cells was counted in each smear. Qualitative
urine and blood PCR for BKV DNA performed for
patients were positive for presence of decoy
cells in their urine cytology. Moreover, urine
analysis was performed for all patients. Urine
cytology was performed at 3 and 6 months after
the first evaluation. Simultaneously, in order
to assess renal function, serum creatinine was
measured three times. Since GFR is considered
as a highly sensitive and specific scale for
chronic renal failure, it was calculated by
MDRD formula based on serum creatinine. Transplant
kidney biopsy was performed considering medical
indications approved by expert nephrologist
(5).
Statistical analysis
All data were analyzed using the SPSS®23
statistical software package. Quantitative demographic
characteristics are expressed as mean ±
standard deviation (SD) and qualitative data
are shown as a percentage. To compare means
of two normally distributed data, the Student's
t-test was used. For non-normally distributed
data, the Mann-Whitney U-test was used. For
comparisons of the correlations between the
two groups, the chi-square and Fisher's exact
tests were used. A p-value of <0.05 was considered
statistically significant.
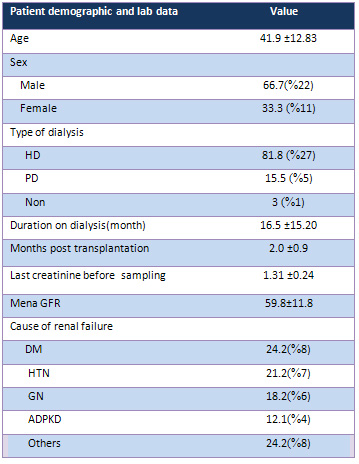
Demographic
data
Thirty-three
patients
(22
male
and
11
female)
received
kidney
transplant
from
living
donors.
The
average
of
patients'
age
was
41.9±12.83
(range:
20-63
years).
Peritoneal
and
hemodialysis
were
used
for
15.6%
and
84.4%
of
recipients.
After
transplantation,
patients
received
prednisone,
cyclosporine,
and
mycophenolate
mofetil.
The
average
of
months
of
interval
between
transplantation
and
the
first
assessment
was
2±0.9
months
(range:
1-4).
Demographic,
clinical
and
para-clinical
information
of
transplant
recipients
are
revealed
in
Table
1.
Table
1:
Demographic,
Clinical
&
Paraclinical
Information
of
Transplant
Recipients
Revealed
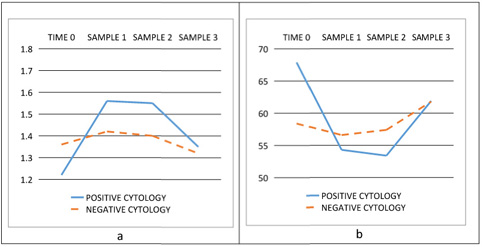
Figure
1:
Plots
of
creatinine
(a)
and
GFR
(b)in
three
consecutive
samplings
Occurrence
of
decoy
cell
viruria
Urine
decoy
cells
were
assessed
in
three
mentioned
intervals
(at
the
time
of
enrollment,
3
and
6
months
later).
Definite
presence
of
decoy
cell,
was
proved
by
qualitative
PCR
of
urine
in
all
cases
with
positive
sample
(Figure
1).
The
occurrence
of
decoy
cell
viruria
at
the
time
of
enrollment,
3
and
6
month
later
was
found
in
18.2%,
10.7%
and
zero,
respectively.
One
case
with
decoy
cell
viruria
and
positive
for
CMV
and
BK-PCR
underwent
renal
biopsy
and
showed
no
viral
changes.
The
number
of
decoy
cells
in
each
high
power
field
is
shown
in
Table
1.
Evaluation
of
renal
function
Serum
creatinine
and
urine
analysis
were
used
for
the
evaluation
of
renal
function.
The
level
of
creatinine
was
1.43±0.29
mg/dL,
1.39±0.24
mg/dL
and
1.35±0.26
mg/dL
in
the
three
steps
of
the
survey.
Also,
estimated
GFR
(eGFR)
was
calculated
using
the
MDRD
formula.
In
three
steps
of
follow
up
the
value
of
eGFR
was
55.3±11.4
mls/min/1.73m2,
57.3±11.7
mls/min/1.73m2
and
61.8±14.3mls/min/1.73m2.
The
urinary
WBC
count
was
8.0±10.4,
7.4±4.1
and
6.7±3.3
in
three
intervals.
Moreover,
the
urinary
count
of
RBC
was
23.3±17.7,
9.2±3.4
and
2.1±1.6
respectively.
Correlation
of
decoy
cell
viruria
and
renal
function
Independent
t-test
demonstrated
that
there
is
a
significant
difference
between
renal
function
and
decoy
cell
viruria
after
2
months
of
follow
up.
(P=
0.017)
Moreover,
the
count
of
RBC
was
significantly
lower
in
patients
with
decoy
cell
viruria
(P=
0.001).
After
5
months
of
follow
up
the
level
of
creatinine
was
significantly
higher
in
patients
with
decoy
cell
viruria
(0.3±0.17
vs
0.2±0.04).
Results
of
Spearman's
rho
test
are
demonstrated
in
Table
2.
Regarding
all
of
the
93
samples
the
level
of
creatinine
was
significantly
higher
in
patients
with
decoy
cel
viruria.
Additionally,
there
was
no
significant
difference
between
occurrence
of
decoy
cell
viruria
and
count
of
WBC
and
RBC.
In
order
to
make
a
better
correlation
between
decoy
cell
viruria
and
renal
function,
we
divided
patients
into
two
groups,
including
GFR
lower
than
60
(group
A)
and
larger
and
equal
to
60
(group
B).
The
average
of
patients'
age
in
group
A
was
significantly
higher
than
group
B
(46.2±11.5
vs
36.2±12,
P<0.001).
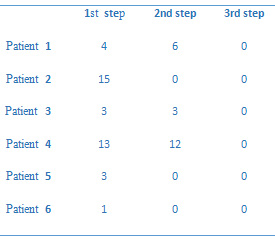
Table
2:
Decoy
Cell
Per
Smear
The
GFR
of
groups
A
and
B
were
65±13
and
56±9
respectively
and
they
were
significantly
different.
Male
patients
were
significantly
more
in
group
A
rather
than
females.
89%
of
patients
with
positive
decoy
cell
viruria
were
in
group
A,
while
60.2%
of
patients
without
decoy
cell
viruria
were
in
this
group
(P=0.039).
Since
there
was
a
significant
correlation
between
post-transplantation
GFR
and
age,
sex
and
pre-transplantation
GFR,
we
used
logistic
regression
test
to
control
their
confounding
effect.
According
to
this,
we
concluded
that
there
was
a
significant
correlation
between
post-transplantation
GFR
and
positivity
for
decoy
cell
viruria
(OR=11.6;
95%
CIs
1.12-120.04,
p=0.02)
(Figure
2).
One
of
the
leading
causes
of
graft
loss
after
kidney
transplantation
is
polyomavirus.
JC
and
BK
virus
infection
is
very
prevalent
in
the
first
two
years
after
transplant
and
might
be
monitored
appropriately
(6).
Routine
screening
for
BK
has
been
shown
to
be
effective
in
preventing
allograft
loss
in
patients
with
BK
viruria
or
viremia.
Reduction
of
immunosuppression
remains
the
mainstay
of
BK
nephropathy
treatment
and
is
the
best
studied
intervention
(7).
The
present
study
was
conducted
on
thirty-three
patients
(22
males
and
11
females)
who
received
kidney
transplantation
from
living
donors.
The
average
of
patients'
age
was
41.9±12.83
(range:
20-63)
years.
In
a
similar
study
in
Iran
thirty-one
patients
(21
men
and
10
women)
received
kidney
transplant
from
living
donors.
In
this
study,
the
average
of
patients'
age
was
38.3±12.8
(rage:
17-59)
years
(8).
Urine
cytology
is
a
safe,
noninvasive
and
sensitive
tool
for
the
evaluation
and
follow-up
of
renal
transplant
recipients
and
can
be
used
as
prospective
screening
for
BKV
allograft
nephropathy
(9).
In
BKV
nephropathy,
the
finding
of
urinary
decoy
cells
showed
a
100%
sensitivity,
84%
specificity,
100%
negative
predictive
value
and
6%
positive
predictive
value
(10).
In
the
first
step
of
follow
up
of
our
study,
the
presence
of
decoy
cells
was
18.2%
in
kidney
transplant
recipients,
while
another
report
demonstrated
the
presence
of
decoy
cells
in
37.5%
of
patients
(8).
In
this
study,
the
occurrence
of
decoy
cell
viruria
at
the
time
of
enrollment,
and
3
and
6
months
later
was
18.2%,
10.7%
and
zero,
respectively.
Moreover,
in
another
study
the
prevalence
of
polyoma
virus
infections
increased
with
increasing
time
after
transplantation
(11),
which
is
similar
to
the
study
by
Liu
et
al.
(12)
Another
same
study
revealed
that
Urinary
decoy
cell
shedding
was
detected
in
26.2%
of
286
cases.
BKV
viruria
was
observed
in
22.1%
of
938
cases
and
BKV
viremia
in
5.2%
of
1,029
cases.
(13)
One
study
in
2007,
presented
that
significant
polyoma
viruses
viruria
is
common
following
renal
transplantation
with
onset
usually
within
the
first
3
months.
Viruria
is
associated
with
worse
graft
function
at
3
and
6
months.
The
time
between
urine
positivity
and
clinical
polyoma
virus
nephropathy
is
short.
More
frequent
early
urine
screening
would
be
required
to
achieve
clinical
benefit.
In
another
study,
the
incidences
of
viruria
and
viremia
at
1
year
were
35%
and
11.5%,
respectively,
compared
with
17%
and
3%
at
a
time
of
49
months
post-transplantation.(10)
Although
managing
a
BKV
infection
includes
reducing
immunosuppression
alone
or
combined
with
antiviral
therapy,
such
as
cidofovir
or
leflunomide,
only
an
early
diagnosis
and
reduction
of
immunosuppression
reliably
improve
graft
survival.
(13)
The
present
study
also
revealed
that
the
level
of
plasma
creatinine
was
significantly
higher
in
patients
with
decoy
cell
viruria.
This
correlation
is
similar
to
another
study
demonstrating
that
patients
with
BK-virus
nephropathy
had
high
serum
creatinine
that
mimicked
either
tubular
necrosis
or
rejection
(14).
The
same
study
in
2006,
suggested
that
the
presence
of
decoy
cells
along
with
high
creatinine
is
a
better
indicator
of
the
virus
presence.
According
to
this
study,
there
was
a
significant
correlation
between
post-transplantation
GFR
and
positivity
for
decoy
cell
viruria.
Despite
a
low
positive
predictive
value
of
decoy
cells
in
urine,
its
absence
has
a
negative
predictive
value
of
100%,
because
almost
all
of
those
patients
who
did
not
have
decoy
cells
had
normal
renal
function.
In
conclusion,
our
findings
suggest
that
considering
the
risk
of
graft
loss
due
to
polyoma
virus
infection,
routine
urine
cytology
might
be
used
as
a
screening
method
for
the
detection
of
polyoma
virus
infection.
As
urine
cytology
is
easy
to
perform
and
of
low
cost,
it
is
a
useful
tool
for
the
investigation
of
active
polyoma
virus
infection.
Moreover,
the
findings
advocate
that
the
presence
of
decoy
cells
along
with
high
creatinine
is
a
better
indicator
of
the
virus
presence
(15).
1.
Salvatore
SP,
Myers-Gurevitch
PM,
Chu
S,
Robinson
BD,
Dadhania
D,
Seshan
SV.
Polyoma
(BK)
virus
associated
urothelial
carcinoma
originating
within
a
renal
allograft
five
years
following
resolution
of
polyoma
virus
nephropathy.
Clinical
nephrology.
2016;85(3):179-83.
2.
Esmaili
H,
Mostafidi
E,
Ardalan
M,
Vahedi
A,
Mahmoodpoor
F,
Mohajel-Shoja
M.
BK
virus
nephropathy
is
not
always
alone.
Journal
of
renal
injury
prevention.
2016;5(1):12-6.
3.
Petrov
R,
Elbahloul
O,
Gallichio
MH,
Stellrecht
K,
Conti
DJ.
Monthly
screening
for
polyoma
virus
eliminates
BK
nephropathy
and
preserves
renal
function.
Surgical
infections.
2009;10(1):85-90.
4.
Ghafari
A,
Lessan-Pezeshki
M,
Taghizadieh
M,
Rahimi
E.
BK
polyoma
virus
nephropathy
among
Iranian
renal
transplant
recipients.
Transplantation
proceedings.
2008;40(1):193-5.
5.
Nesselhauf
N,
Strutt
J,
Bastani
B.
Evaluation
of
leflunomide
for
the
treatment
of
BK
viremia
and
biopsy
proven
BK
nephropathy;
a
single
center
experience.
Journal
of
nephropathology.
2016;5(1):34-7.
6.
Taheri
S,
Kafilzadeh
F,
Shafa
M,
Yaran
M,
Mortazavi
M,
Seirafian
S,
et
al.
Comparison
of
polyomavirus
(BK
virus
and
JC
viruses)
viruria
in
renal
transplant
recipients
with
and
without
kidney
dysfunction.
Journal
of
research
in
medical
sciences
:
the
official
journal
of
Isfahan
University
of
Medical
Sciences.
2011;16(7):916-22.
7.
Koh
MJ,
Lim
BJ,
Noh
S,
Kim
YH,
Jeong
HJ.
Urinary
decoy
cell
grading
and
its
clinical
implications.
Korean
journal
of
pathology.
2012;46(3):233-6.
8.
Pezeshgi
A,
Ghods
A,
Keivani
H,
Asgari
M,
Shatty
M.
Incidence
of
BK
Virus
Nephropathy
(BKVN)
in
Renal
Transplant
Recipients.
International
journal
of
organ
transplantation
medicine.
2012;3(3):115-8.
9.
Jouve
T,
Rostaing
L,
Malvezzi
P.
Place
of
mTOR
inhibitors
in
management
of
BKV
infection
after
kidney
transplantation.
Journal
of
nephropathology.
2016;5(1):1-7.
10.
Vidas
Z,
Misic
M,
Pacic
A,
Jurenec
F,
Knotek
M,
Kardum-Skelin
I.
The
value
of
urinary
decoy
cells
finding
in
patients
with
kidney
transplantation.
Collegium
antropologicum.
2010;34(1):153-7.
11.
Geramizadeh
B,
Roozbeh
J,
Malek-Hosseini
SA,
Azarpira
N,
Ayatollahi
M,
Salahi
H,
et
al.
Urine
cytology
as
a
useful
screening
method
for
polyoma
virus
nephropathy
in
renal
transplant
patients:
a
single-center
experience.
Transplantation
proceedings.
2006;38(9):2923-5.
12.
Liu
LH,
Fresco
R,
Picken
MM.
Pathologic
quiz
case.
Intranuclear
inclusions
in
allograft
kidney.
Pathologic
diagnosis:
human
polymavirus-associated
interstitial
nephritis
in
the
allograft
kidney.
Archives
of
pathology
&
laboratory
medicine.
2001;125(7):973-5.
13.
Thamboo
TP,
Jeffery
KJ,
Friend
PJ,
Turner
GD,
Roberts
IS.
Urine
cytology
screening
for
polyoma
virus
infection
following
renal
transplantation:
the
Oxford
experience.
Journal
of
clinical
pathology.
2007;60(8):927-30.
14.
Hirsch
HH,
Steiger
J.
Polyomavirus
BK.
The
Lancet
Infectious
diseases.
2003;3(10):611-23.
15.Koshy
PJ,
Tripathy
A,
Vijayan
M,
Nair
S,
Yuvaraj
A,
Natarajan
G,
et
al.
A
multicentre
study
of
the
spectrum
of
histopathological
changes
in
renal
allograft
biopsies
over
a
period
of
nine
years
from
South
India.
Immunopathol
Persa.
2017;3(1):e05.
|
|
.................................................................................................................

|
| |
|

