Table
1
:
Demographic
data
of
the
studied
group
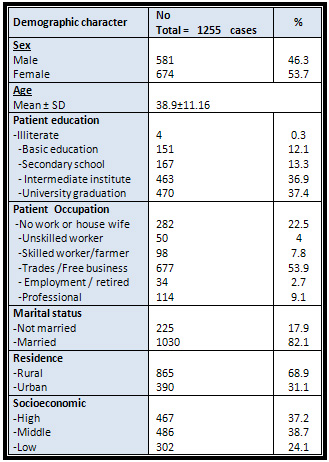
The
total
study
sample
was
1,255
subjects,
46.3%
males
and
53.7
%
females.
The
mean
age
was
38.9±11.16
,
68.9%
of
the
studied
group
were
rural
residents
and
31.1
were
urban
residents
(Table
1).
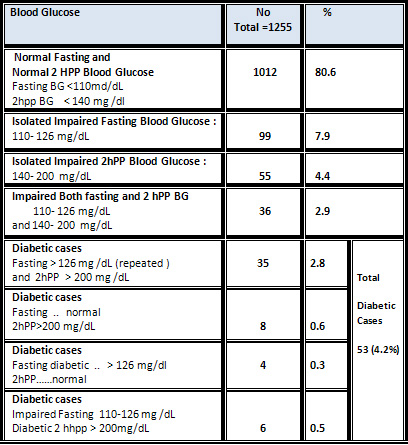
Normoglycemic
individuals
constituted
80.6
%
of
the
studied
group
(normal
fasting
and
2
hours
post
prandial),
while
the
prevalence
of
isolated
impaired
fasting
blood
glucose
was
7.9
%
and
undiagnosed
diabetes
in
patients
who
were
unaware
of
their
glycemic
status,
constituted
4.2%
of
the
studied
group
(2.8
%
had
both
diabetic
fasting
and
2hpp,
0.6%
had
only
diabetic
2hpp
BG
,
0.3%
had
only
diabetic
fasting
BG
and
0.5
%
had
diabetic
2hpp
and
impaired
fasting
BG).
[Table
2
]
&
Figure
[1].
Table
2
:
Distribution
of
the
Studied
Group
According
to
their
Blood
Glucose
Level
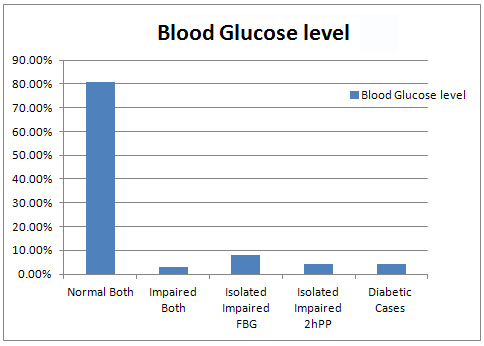
Figure
1:
Distribution
of
Cases
according
to
their
Blood
Glucose
Level
Age
constituted
statistical
significant
difference
and
risk
factor
between
normal
and
IFG
groups,
with
the
highest
prevalence
among
>45
years
(p
<0.001).
Education
of
participants
constituted
statistically
significant
difference
between
the
studied
groups.
Higher
education
grades
seem
to
be
protective
as
the
highest
percentage
of
normal
group
had
university
graduation
(40.7%
)
versus
9.1%
for
the
IFG
group
(p
<0.001)
in
the
normal
fasting
BG
group.
Sex,
occupation,
marital
Status
and
residence
didn't
constitute
statistical
significant
difference
between
the
studied
groups
[Table
3].
Click
here
for
Table
3
:
Comparison
of
Normal
and
Impaired
Fasting
Blood
Glucose
groups
as
Regards
Their
Demographic
Characters
History
of
hypertension
,
hyper-lipidemia
and
viral
hepatitis
were
significantly
higher
among
the
IFBG
group
than
the
normal
fasting
blood
glucose
group
(p
value
=0.003,OR=
2.305
,
CI
(1.323-
4.015)
for
hypertension,
p
value
<0.001,OR=
1.079
,
CI
(2.67
-
8.51)
for
hyper-lipidemia
and
p
value
=0.004
,
OR=
2.803
,
CI
(1.354
-
5.804)
for
chronic
viral
hepatitis),
while
history
of
heart
and
renal
diseases
didn't
constitute
significant
difference
among
the
studied
groups.
Regarding
history
of
medications,
consumption
of
anti-hypertensives
and
corticosteroids
were
significantly
higher
among
the
IFBG
group
than
the
normal
fasting
blood
glucose
group.
Smoking
constituted
no
statistical
difference
between
the
IFBG
group
and
normal
fasting
blood
glucose
group
(p
value
=
0.176
,
OR=
0.731
and
CI
(
0.462-1.153).
Presence
of
relatives
with
diabetes
in
first
and
second
grade
constituted
no
statistical
significant
difference
between
IFBG
and
normal
group
[Table
4].
Click
here
for
Table
4
:
Comparison
of
Normal
and
Impaired
Fasting
Blood
Glucose
Groups
as
Regards
their
History
Obese
participants
(BMI
>
30)
were
significantly
higher
in
the
IFBG
group
than
the
normal
group
(69.4
%
in
IFBG
group
versus
45
%
in
normal
group);
IFBG
while
overweight
were
more
among
the
normal
FBG
group.
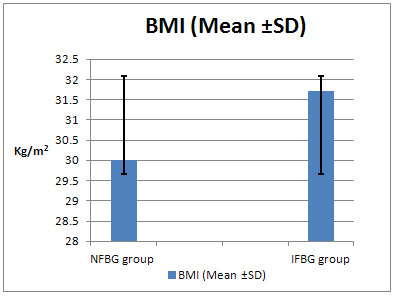
Comparing
the
mean
of
BMI
among
groups
was
significantly
higher
in
IFBG
(p
=0.001)
[Table
5]
[Fig,2].
High
blood
pressure
constituted
12.1
%
of
IFBG
group
versus
5.7
%
in
the
normal
fasting
blood
glucose
group
which
constituted
a
statistical
significant
difference
between
them
(p
value
=
0.002)
[Table
5].
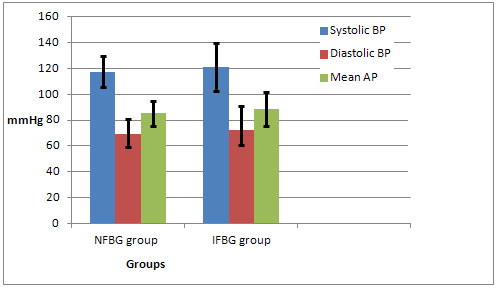
Comparing
means
of
blood
pressure
parameters
(systolic,
diastolic
and
mean
arterial
pressure)
they
were
significantly
higher
in
the
IFBG
group
(P
value
=
0.007,
0.002
and
0.001
respectively)[Table
5]
&[Fig
3]
Table
5
:
Comparison
of
Normal
and
Impaired
Fasting
Blood
Glucose
groups
as
Regards
their
Body
Mass
Index
and
Blood
Pressure
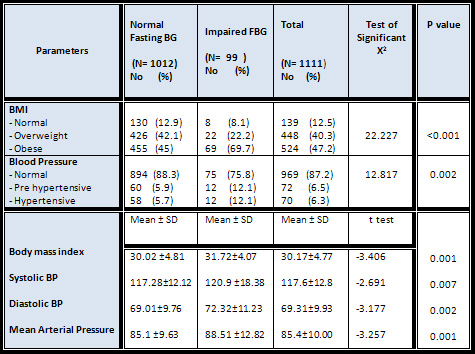
Figure
2:
Comparison
of
BMI
(Mean
±
SD)
between
groups
Figure
3:
Comparison
of
Blood
Pressure
Parameters
(Mean
±
SD)
between
groups
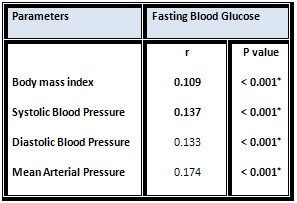
Pearson
correlation
showed
statistical
significant
positive
correlation
of
the
fasting
blood
glucose
values
and
body
mass
index,
systolic,
diastolic
and
mean
blood
pressure
of
the
studied
group
[Table
6
]&[Fig
4,5,6].
Table
6
:
Pearson
Correlation
of
Fasting
Blood
glucose
level
and
(Body
mass
index
and
Blood
Pressure)
parameters
of
the
studied
group
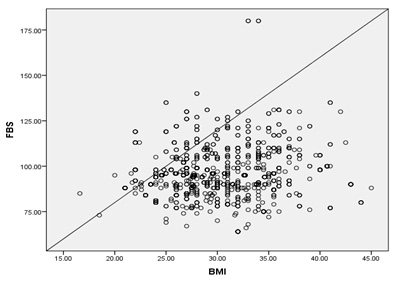
Figure
4
:Pearson
Correlation
of
Fasting
level
and
Body
mass
index
parameters
of
the
studied
group
Figure
5:
Correlation
of
FBG
and
Systolic
blood
pressure
among
the
studied
group
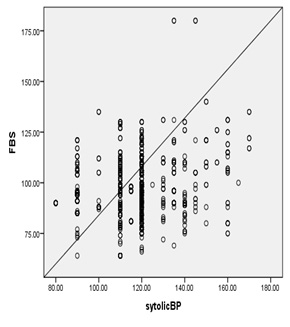
Figure
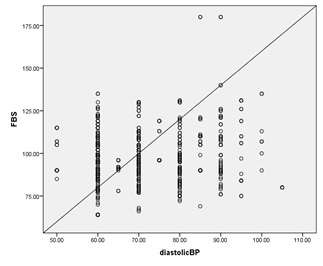
6:
Correlation
of
FBG
and
Diastolic
blood
pressure
among
the
studied
group
Logistic
Regression
Analysis
model
of
risk
factors
associated
with
impaired
fasting
blood
glucose
showed
risk
factors
(including
age,
higher
patient
education,
,
blood
pressure,
receiving
of
anti-hypertensive
medication,
BMI
and
presence
of
diseases
as
hypertension
and
chronic
viral
hepatitis)
were
associated
with
significantly
higher
odds
of
being
in
impaired
fasting
glucose
group.
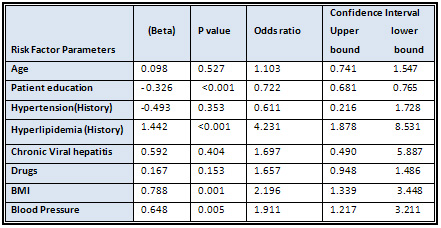
Risk
factors
that
had
the
strongest
prediction
of
impaired
fasting
blood
glucose
were
history
of
hyper-lipidemia
(OR:
4.23,
95%
CIs:
1.87-8.53),
obese
participants
(OR:
2.91,
95%
CIs:
1.21-2.21)
and
patient
education
(OR:
0.722,
95%
CIs:
0.681-0.765)
[Table
7].
Table
7:
Logistic
Regression
Analysis
Of
Risk
Factors
Associated
With
impaired
Fasting
Blood
Glucose
Identification
of
impaired
stages
of
fasting
and
two
hour
post-prandial
blood
glucose
(which
was
given
the
term
pre-diabetes)
is
of
growing
importance
as
interference
through
these
stages
by
modification
of
its
risk
factors
may
delay
the
occurrence
of
type
two
DM
[15
&
16].
So
it
is
important
to
evaluate
this
metabolic
alteration
and
determine
the
main
risk
factors
associated
with
it
in
our
population.
Strict
life
style
changes
and
weight
reduction
is
an
effective
preventive
measure
[17].
This
is
a
cross-sectional
study
and
the
primary
research
question
was
concerned
with
the
prevalence
of
impaired
fasting
glucose
in
Menoufia
governorate,
Egypt.
This
study
reported
the
prevalence
of
undiagnosed
DM
and
impaired
glucose
tolerance
at
4.2%,
and
4.4
%
respectively,
while
the
isolated
impairment
of
fasting
blood
glucose
was
7.9
%
of
the
studied
group.
Lower
estimate
was
reported
in
Latin
America;
the
CARMELA
study
reported
a
prevalence
of
IFG
of
only
2%
[18].
Another
study
in
Taiwan
[19],
had
a
prevalence
of
IFG
so
much
higher
(35.8%),
while
that
of
a
USA
study
was
26%
[20].
In
Venezuela,
prevalence
of
undiagnosed
DM2
was
8.4%
and
that
of
IFG
was
19.5%
of
their
study
population
(2,230
individuals)
[15].
Sinnott
et
al.,
[19]
in
their
screening
study
for
diabetes
and
prediabetes
in
Irish
adults,
reported
a
prevalence
of
IFG
at
7.1%
which
is
nearly
similar
to
our
finding
,
but
they
reported
prevalence
of
undiagnosed
diabetes
at
1.8
%
and
2.9
%
for
impaired
glucose
tolerance
which
is
lower
than
this
study.
They
explained
this
underestimation
of
DM
type2
by
usage
of
Fasting
blood
glucose
only
for
screening.
In
the
current
study,
IFG
was
slightly
higher
in
males
(52.5)
than
females
(47.5),
which
constituted
no
statistical
significant
difference
between
normal
and
impaired
fasting
groups.
This
finding
disagrees
with
studies
done
by
[15,
19],
who
reported
significant
increase
in
males
and
agrees
with
some
other
studies
[20,21]
who
reported
no
significant
difference
between
the
sexes.
Highest
prevalence
of
IFG
was
in
the
age
group
>
45
year
(55.5%),
with
significant
difference
among
normal
and
impaired
fasting
glucose
groups.
This
result
agrees
with
many
studies
[15,
19
and
22].
Studies
attributed
that
to
aging
changes
such
as
waist
circumference
[23],
decreased
lean
mass
[24]
and
diminished
physical
activity
[25].
Atkins
JL
et
al.,
explained
that
by
the
effect
of
aging
on
insulin
resistance
[24].
This
study
reveals
that
higher
education
was
more
prevalent
among
the
normal
fasting
than
the
impaired
fasting
group
(40.7
%
versus
9.1
%).
Education
constituted
a
statistical
significant
difference
among
groups.
Occupation,
socioeconomic
status,
marital
status
had
no
statistically
significant
effect.
Hao
et
al.,
2014
[26],
reported
that
impaired
fasting
glucose
was
prevalent
among
those
of
high
socio-economic
status
in
eastern
China.
Some
studies
reported
no
significant
association
between
IFG
and
socio-economic
standard
of
studied
participants
[15,22].
Aktar
et
al.,
[21]
observed
a
positive
association
of
educational
level
and
socioeconomic
standard
with
diabetes.
In
contrast,
another
study
in
China
reported
that
the
prevalence
of
diabetes
was
generally
unaffected
by
educational
level
but
was
higher
in
the
high-income
group
[27].
Many
studies
reported
that
low
prevalence
of
diabetes
in
better
educated,
highly
socioeconomic
status
group
may
be
due
to
high
health
conscious
level
[28
,
29].
Diaz-Redonodo
et
al.,[6]
reported
that
regarding
risk
factors
of
pre-diabetics,
no
statistically
significant
differences
were
found
in
terms
of
marital
status
or
region
of
residence.
Regarding
history
of
the
patients,
history
of
hypertension
(OR=2.305,
95%
CI
1.323
-
4.015),
hyper-lipidemia
(OR=4.77,95%
CI
2.67
-
8.51)
and
viral
hepatitis
(OR=2.803,95%
CI
1.354
-
5.804)
were
statistically
significantly
higher
among
the
IFG
group
than
the
normal
fasting
group.
A
study
reported
hypertensive
subjects
had
a
2.33
times
higher
risk
of
IFG(6).
Bermúdez
et
al.,[15]
found
hypertension
to
be
a
risk
factor
for
IFG,
however
this
co-relation
between
hypertension
and
alteration
of
IFG
depends
on
other
factors
different
from
IR,
such
as
a
certain
level
of
chronic
inflammation
and
oxidative
stress.
Diaz-Redonodo
et
al.,[6]
reported
that
hypertriglyceridemia
and
low
HDL-Cholesterol
levels
were
also
seen
to
be
associated
with
prediabetes.
The
current
study
showed
that
receiving
medication
for
hypertension
and
corticosteroids
was
statistically
significantly
higher
in
the
IFG
group
than
the
other
group
(14.1
%,12.1
versus
5.8%,
4.7
respectively)
with
p
value
=0.001.
This
may
be
attributed
to
that
thiazide
diuretics,
which
are
a
commonly
used
antihypertensive
medication,
could
increase
insulin
resistance,
affect
glucose
utilization,
precipitate
overt
diabetes
and
worsen
diabetes
control
[30].
Blackburn
et
al
.,
2006
[31]
showed
that
there
is
evidence
indicating
that
thiazide
diuretics
and
certain
beta-blockers
exhibit
adverse
glycemic
effects.
Wong
et
al.,
2008
[32],
showed
no
significant
associations
between
antihypertensive
class
and
impaired
fasting
glucose.
The
therapeutic
benefits
of
glucocorticoids
continue
to
expand
across
medical
specialties,
although
the
incidence
of
steroid-induced
or
steroid-exacerbated
diabetes
continues
to
rise
[33].
In
this
study,
history
of
current
smoking,
daily
exercising,
and
relatives
with
diabetes
shows
non
statistically
significant
difference
between
the
IFG
group
and
the
normal
fasting
group.
Some
studies
reported
that
smoking
is
a
risk
factor
for
prediabetes
[34,35],
however
other
studies
didn't
report
an
association
[6,9].
Underestimation
of
positive
family
history
of
diabetes
may
be
due
to
not
enough
information
among
individuals
about
diagnosed
diabetes
cases
among
the
first
degree
relatives,
who
were
not
exposed
to
medical
diagnosis
before.
Although
physical
exercising
is
recommended
by
WHO
for
protection
from
diabetes,
this
study
showed
no
significant
effect
of
it;
other
studies
found
the
same
finding
[6,9].
The
current
study
showed
that
there
is
a
statistically
significant
difference
between
the
normal
and
fasting
blood
glucose
group
regarding
body
mass
index
and
blood
pressure.
This
was
confirmed
by
comparing
the
means;
the
correlation
which
was
positively
increasing
with
increase
of
the
values
of
fasting
blood
glucose.
The
association
between
hypertension
and
prediabetes
has
been
reported
in
many
previous
studies
[6,9,36].
Some
studies
[15,20]
found
similar
results
regarding
significant
association
of
BMI
on
Fasting
blood
glucose.
This
disagrees
with
Sahai
et
al.,
2011[37],
as
the
notable
finding
in
their
study
was
the
significantly
higher
prevalence
of
IFG
among
the
low
body
weight
population,
raising
the
possibility
of
a
higher
prevalence
of
insulin
deficient
state.
In
Logistic
regression
models
of
risk
factors
for
Impaired
Fasting
Glucose
for
the
population
from
Egypt,
the
risk
factors
that
had
the
strongest
prediction
of
impaired
fasting
blood
glucose
were
history
of
hyper-lipidemia,
obese
participants
and
patient
education.
In
the
study
done
by
Bermúdez
et
al.,[15],
evaluation
of
the
correlation
between
risk
factors
in
a
logistic
regression
analysis
revealed
the
presence
of
insulin
resistance
to
be
the
most
tightly
linked
risk
factor
for
IFG
(OR=2.51;
95%CI=1.79-3.52;
p<0.01),
followed
by
age
groups
(?60
years:
OR=2.31;
95%CI=1.23-4.35;
p<0.01).
Another
study
in
multivariate
analysis
revealed
the
odds
of
developing
pre-diabetes
were
1.4
times
more
among
those
who
were
above
the
age
of
45
years
and
1.5
times
more
in
those
who
were
physically
inactive
[22].
Blood
glucose
in
its
impaired
level
is
not
a
rare
event
and
its
identification
in
the
high
risk
group
as
hypertensive
patient,
patients
with
hyperlipidemia
individuals
>
45
years
and
obesity
is
important
to
deal
with
and
not
to
ignore.
Recommendations:
Based
on
the
findings
of
this
study
,
it
is
recommended
that
screening
should
be
done
to
the
high
risk
group
for
impaired
fasting
blood
glucose
as
those
with
age
>
45
year,
with
a
history
of
hypertension
even
if
controlled
with
medications,
hyperlipidemia,
obesity,
sedentary
lifestyle
and
low
educated
individuals.
This
group
should
modify
their
lifestyle
and
strictly
control
their
blood
pressure
to
safeguard
against
type
2
DM.
1.
Tutuncuoglu
P,
Sarac
F,
Saygili
F,
Ozgen
AG,
Yilmaz
C,
Tuzun
M.
Diabetes
and
impaired
glucose
tolerance
prevalences
in
Turkish
patients
with
impaired
fasting
glucose.
Acta
Diabetol.
2008;45(3):151-6.
2.
Aroda
VR,
Ratner
R.
Approach
to
the
patient
with
prediabetes.
J
Clin
Endocrinol
Metab.
2008;93(9):3259-65.
3.
Da
Rocha
Fernandes
J,
Ogurtsova
K,
Linnenkamp
U,
Guariguata
L,
Seuring
T,
Zhang
P,
et
al.
IDF
Diabetes
Atlas
estimates
of
2014
global
health
expenditures
on
diabetes.
Diabetes
Res
Clin
Pract.
2016;117:48-54.
4.
International
Diabetic
Federation
Atlas
:
Prevalence
of
DM
in
Middle
East
and
South
Africa
[Internet].
[updated
2015].
Available
from:
htmlhttp://
www.diabetesatlas.org/resources/2015-atlas.html
5.
American
Diabetes
Association:
Evidence
-based
nutrition
principals
and
recommendations
for
the
treatment
and
prevention
of
diabetes
and
related
complications
(Position
Statement
).
Diabetes
Care
2003;26(10):
51-61.
6.
Diaz-Redondo
A,
Giraldez-Garcia
C,
Carrillo
L,
Serrano
R,
Garcia-Soidan
FJ,
Artola
S,
et
al.
Modifiable
risk
factors
associated
with
prediabetes
in
men
and
women:
a
cross-sectional
analysis
of
the
cohort
study
in
primary
health
care
on
the
evolution
of
patients
with
prediabetes
(PREDAPS-Study).
BMC
Fam
Pract.2015;16:5.
7.
Nathan
DM,
Davidson
MB,
DeFronzo
RA,
Heine
RJ,
Henry
RR,
Pratley
R,
et
al.
Impaired
fasting
glucose
and
impaired
glucose
tolerance:
implications
for
care.
Diabetes
Care.
2007;30(3):753-9.
8.
Grundy
SM.
Pre-diabetes,
metabolic
syndrome,
and
cardiovascular
risk.
J
Am
Coll
Cardiol.
2012;59:635-43.
9.
Khambalia
A,
Phongsavan
P,
Smith
BJ,
Keke
K,
Dan
L,
Fitzhardinge
A,
et
al.
Prevalence
and
risk
factors
of
diabetes
and
impaired
fasting
glucose
in
Nauru.
BMC
Public
Health.
2011;11:719.
10.
Escobedo
J,
Buitrón
LV,
Velasco
MF,
Ramírez
JC,
Hernández
R,
et
al.
(2009)
High
prevalence
of
diabetes
and
impaired
fasting
glucose
in
urban
Latin
America:
the
CARMELA
Study.
DiabetMed
26:
864-871.
11.
Chen
CM
and
Yeh
MC
.The
prevalence
and
determinants
of
impaired
fasting
glucose
in
the
population
of
Taiwan
:
BMC
Public
Health.2013
;13:1123.
12.
El-Gilany
A.,
El-Wehady
A.and
El-Wasify
M.
Updating
and
validation
of
the
socioeconomic
status
scale
for
health
research
in
Egypt.
Eastern?Mediterranean?Health?Journal
2012;18(9):962-68.
13.
James
PA,
Oparil
S,
Carter
BL,
Cushman
WC,
Dennison-Himmelfarb
C,
et
al.
evidence-based
guideline
for
the
management
of
high
blood
pressure
in
adults:
report
from
the
pane
l
members
appointed
to
the
Eighth
Joint
National
Committee
(JNC
8).
JAMA
(2014);311:507-520.
14.
American
Diabetes
Association.
Diagnosis
and
Classification
of
Diabetes
Mellitus.
Diabetes
Care
(2014);37:
81-90.
15.
Bermúdez
V,
Salazar
J,
González
R,
Ortega
A,
Calvo
M,
Olivar
LC,
Morillo
J,
Miquilena
E,
Chávez-Castillo
M,
Chaparro
R,
Cabrera
M
and
Rojas
J.
Prevalence
and
Risk
Factors
associated
with
Impaired
Fasting
Glucose
in
Adults
from
Maracaibo
City,
Venezuela.
J
Diabetes
&
Metabolism.2016;
7:683.
16.
Capes
S,
Anand
S.
What
is
type
2
diabetes?
In:
Gerstein
HC,
Haynes
RB,
eds.
Evidence-based
diabetes
care.
Hamilton,
Ont.;
Decker,
2001:151-63.
17.
Kosaka
K,
Noda
M,
Kuzuya
T.
Prevention
of
type
2
diabetes
by
lifestyle
intervention:
a
Japanese
trial
in
IGT
males.
2005;67(2):152-62.
18.
CDC
(2011)
National
diabetes
factsheet:
national
estimates
and
general
information
on
diabetes
and
prediabetes
in
the
United
States.
Atlanta,
GA:
US
Department
of
Health
and
Human
Services
22:
13-27
19.
Sinnott
M,
Kinsley
BT,
Jackson
AD,
Walsh
C,
O'Grady
T,
Nolan
JJ,
et
al.
Fasting
plasma
glucose
as
initial
screening
for
diabetes
and
prediabetes
in
Irish
adults:
The
Diabetes
Mellitus
and
Vascular
health
initiative
(DMVhi).
PLoS
One.2015;10(4):
e0122704
20.
Kufe
CN,
Klipstein-Grobusch
K,
Leopold
F,
Assah
F,
Ngufor
G,
Mbeh
G,
et
al.
Risk
factors
of
impaired
fasting
glucose
and
type
2
diabetes
in
Yaounde,
Cameroon:
a
cross
sectional
study.
BMC
Public
Health
2015;15:59.
21.
Akter
S,
Rahman
MM,
Abe
SK,
Sultana
P.
Prevalence
of
diabetes
and
prediabetes
and
their
risk
factors
among
Bangladeshi
adults:
a
nationwide
survey.
Bull
World
Health
Organ.
2014;
92(3):204-13,
13A.
22.
Dasappa
H.,
Fathima
FN,
Prabhakar
R
and
Sar
S.
Prevalence
of
diabetes
and
pre-diabetes
and
assessments
of
their
risk
factors
in
urban
slums
of
Bangalore.
J
Family
Med
Prim
Care.2015;
4(3):399-404.
23.
Michalakis
K,
Goulis
DG,
Vazaiou
A,
Mintziori
G,
Polymeris
A,
Abrahamian-Michalakis
A.
Obesity
in
the
ageing
man.
Metabolism.
2013
;
62(10):1341-9.
24.
Atkins
JL,
Whincup
PH,
Morris
RW,
Wannamethee
SG.
Low
muscle
mass
in
older
men:
the
role
of
lifestyle,
diet
and
cardiovascular
risk
factors.
J
Nutr
Health
Aging.
2014;18(1):26-33.
25.
Leon-Latre
M,
Moreno-Franco
B,
Andres-Esteban
EM,
Ledesma
M,
Laclaustra
M,
Alcalde
V,
et
al.
Sedentary
lifestyle
and
its
relation
to
cardiovascular
risk
factors,
insulin
resistance
and
inflammatory
profile.
Rev
Esp
Cardiol
(Engl
Ed).
2014;
67(6):449-55.
26.
Hao
C1,
Zhang
C,
Chen
W,
Shi
Z:Prevalence
and
risk
factors
of
diabetes
and
impaired
fasting
glucose
among
university
applicants
in
Eastern
China:
findings
from
a
population-based
study.
Diabet
Med.
2014;31(10):1194-8.
27.
Hu
FB,
Manson
JE,
Stampfer
MJ
et
al.
Diet,
lifestyle,
and
the
risk
of
type
2
diabetes
mellitus
in
women.
N
Engl
J
Med.
(2001);7:345:790.
28.
Duc
Son
LN,
Kusama
K,
Hung
NT,
Loan
TT,
Chuyen
NV,
Kunii
D,
et
al.
Prevalence
and
risk
factors
for
diabetes
in
Ho
Chi
Minh
City,
Vietnam.
Diabet
Med.
2004;21(4):371-6.
29.
Pan
XR,
Yang
WY,
Li
GW,
Liu
J.
Prevalence
of
diabetes
and
its
risk
factors
in
China,
1994.
National
Diabetes
Prevention
and
Control
Cooperative
Group.
Diabetes
Care.
1997
;20
(11):1664-9.
30.
Mugo
MN,
Link
D,
Stump
CS,
Sowers
JR:
Insulin
Resistance
and
Diabetes
in
Hypertension.
In:
Lip
GYH,
Hall
JE,
editor.
Comprehensive
Hypertension.
Mosby,
Inc;
(2007)
;
682.
31.
Blackburn
DF,
Pharm
D
and
Wilson
TW
:
Antihypertensive
medications
and
blood
sugar:
Theories
and
implications.
Can
J
Cardiol.
2006
;
22(3):
229-233.
32.
Wong
MCS,
Jiang
J
Y,
Fung
H,
Griffiths
S,
and
Mercer
S
:Antihypertensive
drug
class
and
impaired
fasting
glucose:
a
risk
association
study
among
Chinese
patients
with
uncomplicated
hypertension.
BMC
Clin
Pharmacol.(
2008);
8:
6.
33.
Hwang
JL
and
Weiss
R
E.:
Steroid-induced
diabetes:
a
clinical
and
molecular
approach
to
understanding
and
treatment.
Diabetes
Metab
Res
Rev.
2014;
30(2):
96-102.
34.
Willi
C,
Bodenmann
P,
Ghali
WA,
Faris
PD,
Cornuz
J.
Active
smoking
and
the
risk
of
type
2
diabetes:
a
systematic
review
and
meta-analysis.
Jama.
2007
12;
298(22):2654-64.
35.
Vlassopoulos
A,
Lean
MEJ,
Combet
E.
Influence
of
smoking
and
diet
on
glycated
haemoglobin
and
"pre-diabetes"
categorisation:
a
cross-sectional
analysis.
BMC
Public
Health.
2013;13:1013.
36.
Heianza
Y,
Hara
S,
Arase
Y,
Saito
K,
Fujiwara
K,
Tsuji
H,
Kodama
S,
Hsieh
SD,
Mori
Y,
Shimano
H,
Yamada
N,
Kosaka
K,
Sone
H.
HbA1c
5.7-6.4%
and
impaired
fasting
plasma
glucose
for
diagnosis
of
prediabetes
and
risk
of
progression
to
diabetes
in
Japan
(TOPICS
3):
a
longitudinal
cohort
study.
Lancet.
2011
;
378:147-55.
37.
Sahai
S,
Vyas
D,
Sharma
S.
Impaired
Fasting
Glucose:
A
Study
of
its
Prevalence
Documented
at
a
Tertiary
Care
Centre
of
Central
India
and
its
Association
with
Anthropometric
Variables.
JIACM
2011;
12:
187-192.

