|
The
Prevalence of Diabetic Retinopathy and associated Risk
Factors in Type 2 Diabetes Mellitus in Al-Naeem area
(Kuwait)
|
|

Authors:
Dr. Farhan KH Al-Shammari
Dr. Osama Al-Meraghi
Dr. Alfred Nasif
Dr. Saad Al-Otaibi
Correspondence:
Dr. Farhan KH Al-Shammadi
Al Jahra Heaith Area, Al-neeam Clinic,
Kuwait State
Email: fkks69@hotmail.com

ABSTRACT
Background
Studying the prevalence of diabetic retinopathy and associated
risk factors in type II diabetes mellitus in an Al-naeem area
(Kuwait) case control study comparing type II diabetic with
retinopathy and without retinopathy after group matching for
age and sex to control confounders.
Results
HbA1c (p=0.0001), microalbuminuria (p=0.0001), neuropathy
(p=0.002), insulin treatment (p=0.0001), body mass index >30(p=0.0001)
and diabetes duration (p=0.0001); are significant risk factors
in this study. On the other hand; age at diagnosis, total
cholesterol, low density lipoprotein, triglycerides, and duration
of hypertension are significant in univariate analysis where
as hypertension, smoking and family history of diabetic retinopathy
are insignificant
Methods
698 DM type II patients, 349 have retinopathy and 349 without
retinopathy, 352 females and 346 males were identified and
group matched for age and sex in a Al-naeem clinic in Jahra
area (Kuwait).
Conclusion
This study shows that type II diabetes with poor control of
blood sugar, longer diabetes duration, nephropathy, on insulin
treatment and body mass index >30 are more prone to develop
retinopathy.
BACKGROUND
Retinopathy can be found in as many as three-quarters
of individuals who have had diabetes mellitus for more than
15 years[1]. Diabetic retinopathy is a leading cause of visual
impairment among diabetic (DM) people. Numerous studies have
investigated prevalence and risk factors for diabetic retinopathy
(DR) but few case control studies in DM. This case control
was conducted over 20 months (May 2003 till December 2004).
In this study we report the prevalence and risk factors of
retinopathy among type II DM patients in Al-naeem area. Type
II DM patients comprise the greater proportion of diabetics
in Al-naeem area and identification of visual status is relevant
to their care and service provision. Al-naeem clinic is serving
a population of 79952 according to the civil identification
authority and it is an example of other clinics in the Jahra
governorate in Kuwait. 3522 patients with type II DM were
discovered, of which 349 had retinopathy.
The diabetes center in this area started service
in September 2001. DM patients who have DR and patients who
do not have DR (control) were identified by non-mydratic ophthalmoscopic
camera, ophthalmoscopic fundus examination through dilated
pupils and conformation referral to ophthalmologists and all
participants consented to participate in the study. The diagnosis
of type 2 diabetes was based on clinical characteristics that
included 1) diagnosis of diabetes after 30 years of age; and
2) treatment by diet or oral hypoglycaemic agents or insulin
treatment. A diagnosis of diabetic retinopathy was made only
where a participant had a minimum of one microaneurysm in
any field, as well as exhibiting hemorrhages (dot, blot, or
flame shaped), and maculopathy (with or without clinically
significant oedema). All participants (patient & control)
underwent a medical history, blood pressure (BP), height,
weight measurement (by which body mass index (BMI) was calculated),
and total cholesterol (CH), high density lipoprotein (HDL),
low density lipoprotein (LDL), triglycerides (TG), microalbumin
(MIC) in urine, glycosylated haemoglobin (HBA1C) and for conformation,
the investigation done twice except for nine patients who
refused to have height, weight measurements and tests for
microalbuminuria. Competing arterial blood pressure was measured
with a mercury sphygmomanometer in the sitting position after
a 10-min rest. Serum glucose, triglycerides, and total cholesterol
Levels were measured using an autoanalyzer with enzymatic
technique. HbA1C was measured by affinity chromatography (Isolab,
Akron, OH) (normal range 4-8%). Patients collected timed overnight
urine samples for the determination of albumin excretion rate
(AER) by radio immunoassay (Diagnostic Products, Los Angeles,
CA). Microalbuminuria was defined as AER 20-200 µg/min.
Serum LDL levels were measured by one step sandwich enzyme-linked
immunosorbent assay using monoclonal antibodies (Immuno, Vienna).
Patients who had paraesthesia in lower limbs and had treatment
for it (anticonvulsant) are considered as neuropathic. The
multivariate logistic analysis was performed; the dependent
variable was no DR (0) .DR (1). The independent variables
(covariates) were CH, HDL, LDL, MIC, HBA1C, BMI>30,TG,
smoking, age at diagnosis (AG), hypertension (HPN), duration
of hypertension (DH), insulin treatment (INT).
RESULTS
The prevalence of retinopathy in type 2 diabetes
was 12/100. For DR patient, the means of age at diagnosis
(41.1±9.2), age (54.28±8.7), duration of diabetes
(13.2±5.8), duration of hypertension (4.6±6.6),
family history of diabetes mellitus (0.55±0.5), cholesterol
(5.65±1.2)), hba1c (11.5±9.8), ldl (3.6±1.1),
microalbuminuria (15.1±30.2), hypertension (.44±0.5),
neuropathy (0.2±0.4), insulin treatment (0.61±0.49),
triglycerides (2.3±1.4) Body mass index >30 (0.45±0.5)
and smoking (0.21±0.41); whereas for patients without
DR the mean of age at diagnosis (47.1±9.2), age (54.09±8.5),
sex (0.5±0.5), duration of diabetes (7±5.23),
duration of hypertension (3.05±5), family history of
diabetes mellitus (0.49±0.50), cholesterol (5.32±1.05),
hba1c (8.4±2.8), ldl (3.4±0.9), microalbuminuria
(6.55±12.6), hypertension (.0.38±0.49), neuropathy
(0.06±0.23), insulin treatment (0.18±(0.39),
triglycerides (2.19±1.3) body mass index >30 (0.55±0.5)
and smoking (0.24±0.43); are shown in table 1. The
univariate analysis, the p-value , also presented in table
1 is as follows: age at diagnosis (0.0001), age (1), sex (1),
confounders controlled by age and sex group matching, duration
of diabetes (0.0001), duration of hypertension (0.0001), hba1c
(0.0001), ldl (0.002), microalbuminuria (0.001), cholesterol
(0.0001), neuropathy (0.0001), insulin treatment (0.0001),
triglyceride (0.035), body mass index >30 (0.01) family
history of diabetic retinopathy (insignificant), hypertension
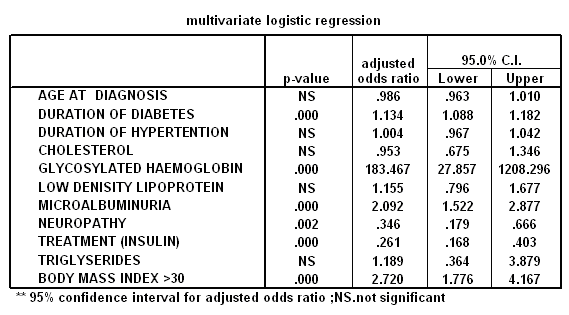
(insignificant), and smoking (insignificant). The multivariate
logistic analysis shown in table 2 where; HbA1c (0.0001),
microalbuminuria (0.0001), neuropathy (0.002), insulin treatment
(0.0001), body mass index >30(0.0001) and diabetes duration
(0.0001); are the only significant risk factors in this study.
DISCUSSION
The prevalence in this study was of no difference
to other populations[2-4]
After controlling age and sex during the comparison
between diabetics who had DR and who had not, we found that
poor blood sugar control (high HbA1C), obesity (BMI>30),
nephropathy (MIC), neuropathy, INT and longer duration of
diabetes mellitus are the risk factors for DR and it seems
convincing that diabetics who had these risk factors are more
borne to develop DR.
This study also showed other risk factors like
age at diagnosis, total cholesterol, triglycerides, LDL, duration
of hypertension which are relevant in univariate analysis,
whereas a family history of diabetic retinopathy, hypertension
and smoking are irrelevant. Poor blood sugar control indicated
by high HbA1C has been found as a risk factor by other studies[5-12].
Nephropathy was also found as a risk factor[2, 8, 11, 13-17]
as was duration of diabetes supported by these studies [2-4,
6, 8, 13, 16-22] Insulin treatment was found by studies like[6,
12, 13, 21-23] . Compared to studies[11, 23, 24] who found
lower body mass index is more associated with DR and to our
study which found body mass index >30 as a risk factor
that may be explained by the difference between the population
and the study design, where BMI analysed as dichotomous variable
and controlling age and sex might lead to this discrepancy
in results, on the contrary was not found as a risk factor[22,
25]. Concerning neuropathy, to our knowledge there is a study
[26]that has investigated it and it interpreted that diabetics
who had neuropathy are more likely to develop DR and should
be sent to an ophthalmologist since 87% of the patients with
retinopathy had signs of peripheral neuropathy.
Although the study conducted in one center was considered
to have limitations, being the centre having the highest score
given by the Kuwait diabetes committee which is responsible
for the application of Kuwait guidelines for diabetes, still
we can not determine AG, DH, LDL, CH, TG, family history of
DR, HPN, and smoking as risk factors.
AG was shown by [6, 27] both studies comparing
different ethnic group which is not the case in this study.
As far as we know there is no study trying to find an association
between DH and family history of DR and this association cannot
be indicated by this study.
We can not prove that CH, LDL, TG are associated
with DR and this is shown also by[9, 20, 22, 28].On the other
hand other studies prove CH as a risk factor and this is explained
by the difference in the sample selected where type I diabetics
were included[18] whereas [23] a study the population of Cree
Indians of James Bay which may be different from our population.
LDL was shown as a relevant risk factor for
patients who have proliferative DR[29] and this can be explained
as the sample population was different from the sample of
our population in which they divide their sample into three
groups, DM without DR, nonproliferative DR and proliferative
DR.
Although HPN found as a risk factor by [20,
30] which compares diabetics with non diabetics and hypertensive
diabetics with normotensive diabetics respectively.[3] also
found HPN relevant. Other studies found the opposite[13] which
is a screening study. All such studies did not control the
age and the sex by group matching.
Cigarette smoking is not a risk factor for retinopathy
[22, 25, 31]and this support our finding where as study [3]
found marginal effect of smoking relevant. However, the failure
of ours to find an association between smoking and diabetic
retinopathy does not imply that persons with diabetes who
do not smoke should start smoking as cigarette smoking is
a risk factor for other complications and associated conditions
of diabetes, particularly cardiovascular disease.
CONCLUSION
In this case control study we investigated the
prevalence of retinopathy in type II diabetic in Al-naeem
clinic which is comparatively consistent with other population
prevalence and the risk factors of retinopathy. It has been
shown that poor control of blood sugar, longer diabetes duration,
microalbuminuria, insulin treatment and body mass index >30
increase the risk for development of retinopathy. The significant
associations with poor control and duration of diabetes provide
further strong evidence for the benefits of optimal glycaemic
control and body weight reduction. There is a debate about
DR risk factors; further study is needed to define them
ACKNOWLEDGEMENT
Dr Ali Aldaher the head of primary care
department in Aljahra area

REFERENCES
- R Klein, BE Klein, SE
Moss, MD Davis, DL DeMets: The Wisconsin epidemiologic study
of diabetic retinopathy. III. Prevalence and risk of diabetic
retinopathy when age at diagnosis is 30 or more years. Arch
Ophthalmol 1984, 102:527-32.
- RJ Tapp, JE Shaw, CA Harper, MP de Courten,
B Balkau, DJ McCarty, HR Taylor, TA Welborn, PZ Zimmet:
The prevalence of and factors associated with diabetic retinopathy
in the Australian population. Diabetes Care 2003, 26:1731-7.
- JM Sparrow, BK McLeod, TD Smith, MK Birch,
AR Rosenthal: The prevalence of diabetic retinopathy and
maculopathy and their risk factors in the non-insulin-treated
diabetic patients of an English town. Eye 1993, 7 ( Pt 1):158-63.
- EH Sidibe: [Diabetic retinopathy in Dakar
and review of African literature: epidemiologic elements].
Diabetes Metab 2000, 26:322-4.
- O Cohen, K Norymberg, E Neumann, H Dekel:
Complication-free duration and the risk of development of
retinopathy in elderly diabetic patients. Arch Intern Med
1998, 158:641-4.
- SM Haffner, BD Mitchell, SE Moss, MP Stern,
HP Hazuda, J Patterson, WA Van Heuven, R Klein: Is there
an ethnic difference in the effect of risk factors for diabetic
retinopathy? Ann Epidemiol 1993, 3:2-8.
- HC Looker, J Krakoff, WC Knowler, PH Bennett,
R Klein, RL Hanson: Longitudinal studies of incidence and
progression of diabetic retinopathy assessed by retinal
photography in pima indians. Diabetes Care 2003, 26:320-6.
- RM Voutilainen-Kaunisto, ME Terasvirta, MI
Uusitupa, LK Niskanen: Occurrence and predictors of retinopathy
and visual acuity in Type 2 diabetic patients and control
subjects. 10-year follow-up from the diagnosis. J Diabetes
Complications 2001, 15:24-33.
- CH .Kim, HK Kim, SW Kim, JY Park, SK Hong,
YH Yoon, KU Lee: Development and progression of diabetic
retinopathy in Koreans with NIDDM. Diabetes Care 1998, 21:134-8.
- A Sasaki, N Horiuchi, K Hasewgawa, M Uehara:
Development of diabetic retinopathy and its associated risk
factors in type 2 diabetic patients in Osaka district, Japan:
a long-term prospective study. Diabetes Res Clin Pract 1990,
10:257-63.
- HT Nguyen, SD Luzio, J Dolben, J West, L
Beck, PA Coates, DR Owens: Dominant risk factors for retinopathy
at clinical diagnosis in patients with type II diabetes
mellitus. J Diabetes Complications 1996, 10:211-9.
- MC Leske, SY Wu, A Hennis, B Nemesure, L
Hyman, A Schachat: Incidence of diabetic retinopathy in
the Barbados Eye Studies. Ophthalmology 2003, 110:941-7.
- G Phillipov, A Alimat, PJ Phillips, AC Drew:
Screening for diabetic retinopathy. Med J Aust 1995, 162:518-20.
- M Lunetta, L Infantone, AE Calogero, E Infantone:
Increased urinary albumin excretion is a marker of risk
for retinopathy and coronary heart disease in patients with
type 2 diabetes mellitus. Diabetes Res Clin Pract 1998,
40:45-51.
- M Janghorbani, M Amini, H Ghanbari, H Safaiee:
Incidence of and risk factors for diabetic retinopathy in
Isfahan, Iran. Ophthalmic Epidemiol 2003, 10:81-95.
- MR Manaviat, M Afkhami, MR Shoja: Retinopathy
and microalbuminuria in type II diabetic patients. BMC Ophthalmol
2004, 4:9.
- P Luzniak, A Czech, J Taton: [Prospective
studies of diabetic retinopathy in a cohort of patients
with type II diabetes mellitus]. Pol Merkuriusz Lek 1997,
2:14-7.
- AM El-Asrar, KA Al-Rubeaan, SA Al-Amro, D
Kangave, OA Moharram: Risk factors for diabetic retinopathy
among Saudi diabetics. Int Ophthalmol 1998, 22:155-61.
- G Giuffre, G Lodato, G Dardanoni: Prevalence
and risk factors of diabetic retinopathy in adult and elderly
subjects: The Casteldaccia Eye Study. Graefes Arch Clin
Exp Ophthalmol 2004, 242:535-40.
- HA van Leiden, JM Dekker, AC Moll, G Nijpels,
RJ Heine, LM Bouter, CD Stehouwer, BC Polak: Risk factors
for incident retinopathy in a diabetic and nondiabetic population:
the Hoorn study. Arch Ophthalmol 2003, 121:245-51.
- M Cahill, A Halley, M Codd, N O'Meara, R
Firth, D Mooney, RW Acheson: Prevalence of diabetic retinopathy
in patients with diabetes mellitus diagnosed after the age
of 70 years. Br J Ophthalmol 1997, 81:218-22.
- MS Chen, CS Kao, CJ Chang, TJ Wu, CC Fu,
CJ Chen, TY Tai: Prevalence and risk factors of diabetic
retinopathy among noninsulin-dependent diabetic subjects.
Am J Ophthalmol 1992, 114:723-30.
- DA Maberley, W King, AF Cruess, A Koushik:
Risk factors for diabetic retinopathy in the Cree of James
Bay. Ophthalmic Epidemiol 2002, 9:153-67.
- GK Dowse, AR Humphrey, VR Collins, W Plehwe,
H Gareeboo, D Fareed, F Hemraj, HR Taylor, J Tuomilehto,
KG Alberti, et al: Prevalence and risk factors for diabetic
retinopathy in the multiethnic population of Mauritius.
Am J Epidemiol 1998, 147:448-57.
- R McKay, CA McCarty, HR Taylor: Diabetic
retinopathy in Victoria, Australia: the Visual Impairment
Project. Br J Ophthalmol 2000, 84:865-70.
- C Delcourt, B Villatte-Cathelineau, F Vauzelle-Kervroedan,
L Papoz: Clinical correlates of advanced retinopathy in
type II diabetic patients: implications for screening. The
CODIAB-INSERM-Zeneca Pharma Study Group. J Clin Epidemiol
1996, 49:679-85.
- RF Hamman, EJ Mayer, GA Moo-Young, W Hildebrandt,
JA Marshall, J Baxter: Prevalence and risk factors of diabetic
retinopathy in non-Hispanic whites and Hispanics with NIDDM.
San Luis Valley Diabetes Study. Diabetes 1989, 38:1231-7.
- MS Chen, CS Kao, CC Fu, CJ Chen, TY Tai:
Incidence and progression of diabetic retinopathy among
non-insulin-dependent diabetic subjects: a 4-year follow-up.
Int J Epidemiol 1995, 24:787-95.
- CH Kim, HJ Park, JY Park, SK Hong, YH Yoon,
KU Lee: High serum lipoprotein(a) levels in Korean type
2 diabetic patients with proliferative diabetic retinopathy.
Diabetes Care 1998, 21:2149-51.
- M Cignarelli, ML De Cicco, A Damato, A Paternostro,
S Pagliarini, S Santoro, L Cardia, G De Pergola, R Giorgino:
High systolic blood pressure increases prevalence and severity
of retinopathy in NIDDM patients. Diabetes Care 1992, 15:1002-8.
- SE Moss, R Klein, BE Klein: Cigarette smoking
and ten-year progression of diabetic retinopathy. Ophthalmology
1996, 103:1438-42.

TABLE 1
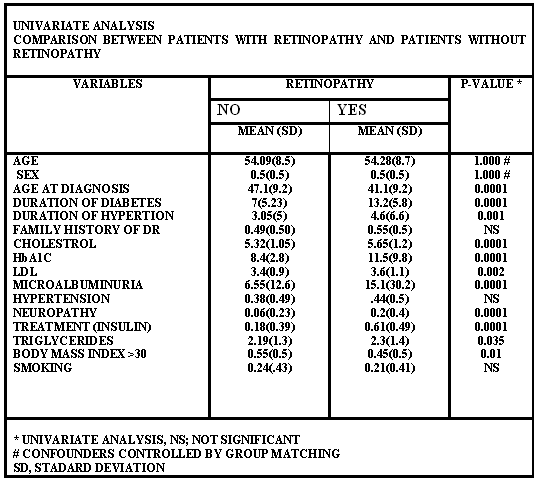
TABLE 2
|


