|
The
Diagnosis and Management of Dementia
|
|
Authors
David G. Clark, M.D.1
Jeffrey L. Cummings, M.D.2
From the Departments of Neurology (1,2),
and Psychiatry and Biobehavioral Sciences (2),
David Geffen School of Medicine at UCLA, Los Angeles, California.
Correspondence
Jeffrey L. Cummings, M.D.
Reed Neurological Research Center
David Geffen School of Medicine at UCLA
710 Westwood Plaza
Los Angeles, CA 90095-1769
Telephone: 310-206-5238
Fax: 310-206-5287
Email: cummings@ucla.edu

The Increasing Burden of Dementing Disease
Dementia is a common, disabling and distressing
neurological disorder and should not be considered a feature
of normal aging. Many people reach old age without developing
disabling cognitive impairment, although some aspects of cognition
routinely change with age. These changes vary among aged individuals
and involve slowing of reaction times and reduction of memory
capacity and visuospatial skills (1). Despite the presence
of measurable neuropsychological alterations in the majority
of elderly people, those who experience these changes typically
retain their ability to conduct their daily social and occupational
activities. These mild alterations stand in stark contrast
to the derangements of cognition that lead to dementia, with
loss of normal function. The proportion of aging individuals
who develop dementia is substantial, and the aging segment
of the population is expanding worldwide.
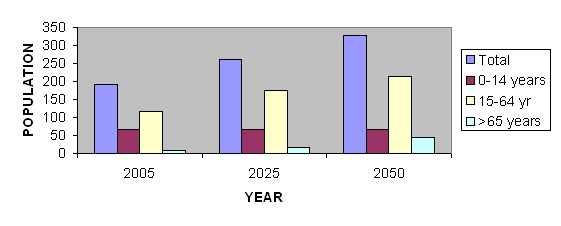
According to World Health Organization (WHO)
estimates, the total population of ten selected Middle Eastern
nations (Algeria, Bahrain, Egypt, Iraq, Israel, Kuwait, Lebanon,
Libya, Saudi Arabia and Syria) will continue to expand through
the first half of this century, exceeding 326 million by the
year 2050. During this interval the proportion of the population
over the age of 65 will grow at a greater rate than other
segments of the populace. Thus, although only 6.2% of the
adult population of these countries is projected to be over
the age of 65 in 2005, this percentage will rise to 17.1%
by the year 2050 (2) (Figure 1). The expansion of the number
of aged individuals in the population will inevitably be accompanied
by an increasing number of persons with dementia and pre-dementia
mild cognitive impairment (MCI). A further concern is that
this will not be accompanied by a comparable increase in the
occupationally productive segment of the population. While
the 15-64 year old age group is projected to increase by 81%,
the over-65 year-old group will increase by 468%.
Diseases that result in cognitive impairment are common and
increase in prevalence with age. The aged segment of the population
is growing rapidly in most countries of the world and high
rates of dementing disease are expected during the next fifty
years. In the United States there were 2.32 million individuals
with Alzheimer's disease (AD) in 1997 and this number is expected
to increase to at least 8.64 million by the year 2050 (3).
The proportion of new AD cases in Middle Eastern nations may
be similar to that of the US, although few studies are available
to guide predictions. Unless a means is found to prevent or
delay the onset of AD, many of the people in the over-65 age
group will become demented, constituting an overwhelming social
and economic burden as well as a personal tragedy and a challenge
to family
Figure 1 Demography of Aging in Ten Middle Eastern
Nations (population in millions)
Mild Cognitive Impairment
Studies of aging that address the epidemiology
of dementia have revealed the presence of three groups of
individuals: those who are cognitively normal, those who are
demented and a third group that have cognitive impairment
but do not meet criteria for dementia. These individuals may
have impairment in a single domain, usually memory. This third
group of patients cannot be classified as "normal"
or as "demented," since the definition of dementia
requires abnormalities in at least two cognitive domains and
social or occupational disability. These individuals have
been labeled as mild cognitive impairment (MCI). The most
clearly characterized form of MCI is known as the "amnestic
form"; these patients are characterized by subjective
memory complaints and evidence objective memory impairment
but have normal cognitive function in other domains and intact
ability to carry out activities of daily living (4)(See Table
1). In research studies, patients typically meet an operational
criterion of 1.5 or more standard deviations below the mean
for age-matched controls on standard neuropsychological tests
of memory (5).
Table 1 Criteria for mild cognitive
impairment (4)
| Memory complaint, preferably corroborated by an informant |
| Objective memory impairment (below 1.5 standard deviations) |
| Normal general cognitive functioning |
| Intact activities of daily living |
| Not demented |
Patients with MCI are at increased risk for
the development of AD. The annual incidence of AD in the general
population ranges from 0.2% among those aged 65-69 years to
3.9% among those aged 85-89, but studies estimate the incidence
rate among patients previously diagnosed with MCI to be between
6 and 25% per year (6). Early recognition of these patients
will become increasingly important as treatments are developed
that delay the transition from MCI to AD. Delaying the onset
of AD by as little as six months will have substantial economic
benefits (3). Several clinical trials are under way to investigate
potential pharmacological treatments for MCI (5). Patients
with other forms of MCI (such as mild changes in more than
one domain or in a single non-memory domain) may be at risk
for other forms of dementia, such as dementia with Lewy bodies,
vascular dementia or frontotemporal dementia (4).
Assessment of Dementia
A number of cognitive instruments have proven
useful for screening patients at risk for dementia. The Mini-Mental
Status Exam (MMSE) is widely used. It is sensitive when scores
are adjusted for age and education (6,7). The validity of
the MMSE in some Arab populations has been investigated and
shown to provide acceptable data (8). Cognitive testing with
a short mental status exam should be supplemented with the
bedside evaluation of memory, language, visuospatial abilities,
and frontal-executive functions. Culturally appropriate versions
of these tasks should be selected. Depression can cause cognitive
changes and patients should be screened with questions about
their mood, tearfulness and suicidal ideation.
In addition to measures of cognitive function,
it is important to identify loss of general function or of
the ability to carry out activities of daily living, such
as bathing, grooming, toileting, eating or more complex activities
expected of aged individuals in their cultural setting. These
facts can be gleaned from the history or by interviewing the
patient's caregiver with structured instruments (9). The clinician
can also gain insight regarding the patient's overall level
of function with global rating scales (9, 10). Such informant-based
scales are useful if the informant is observant.
Every patient with suspected dementia should
undergo a thorough physical and neurologic examination. Medical
illnesses that can result in dementia include thyroid disease,
atherosclerotic vascular disease, collagen-vascular diseases
(such as systemic lupus erythematosus), and infections. Thus,
the clinician must be attentive to the skin for thinning of
hair and eyebrows, spider hemangiomata, palmar erythema, malar
rash or Kaposi's sarcoma. The heart sounds, liver texture
and size, or the presence of fever, hypertension or lymphadenopathy
may also provide important diagnostic clues. Visual field
defects, eye movement abnormalities, facial asymmetry, dysarthria,
focal weakness or spasticity may indicate the presence of
focal brain or brainstem lesions due to stroke, tumor or infectious
diseases such as toxoplasmosis.
Where feasible, patients with a clinical dementia
syndrome should undergo structural brain imaging with noncontrast
computed tomography (CT) or magnetic resonance imaging (MRI)
to evaluate for focal lesions, deep white matter ischemic
changes and regions of atrophy.
Certain laboratory tests are valuable for the
initial screening of patients with cognitive changes. In particular,
thyroid function tests (thyroid stimulating hormone and free
T4) and the vitamin B12 level should be checked in patients
with cognitive complaints. In cases of borderline B12 deficiency
elevated levels of homocysteine and methylmalonic acid enhance
the sensitivity of the B12 level. Patients with risk factors
for HIV should undergo appropriate tests. The prevalence of
venereal syphilis is low outside of urban areas in most Middle
Eastern countries, reducing the utility of routine syphilis
testing. The occurrence of non-venereal, endemic syphilis
(bejel) in rural regions of North Africa and the Arabian peninsula
increases the need for caution when interpreting serological
tests for syphilis (11). There is little evidence that bejel
ever results in neurologic complications. The 14-3-3 protein
is present in higher levels in the spinal fluid of patients
with Creutzfeldt-Jakob disease and can be used to support
the diagnosis in patients whose clinical presentation is consistent
with the disorder (12).
Diagnosis of Dementia
Dementia
The definition of dementia provided in the Diagnostic and
Statistical Manual, 3rd edition, revised (DSM-IIIR) has been
found to have adequate reliability and should be used for
making the diagnosis (13,14). These criteria were not changed
in the 4th edition, and are shown in Table 2.
Table 2 DSM-IV Criteria for dementia
(13)
| Short- and long-term memory impairment |
| Impairment in abstract thinking, judgment,
other higher cortical function or personality change |
| Cognitive disturbance interferes with significantly
with work, social activities or relationships with others |
| These cognitive changes do not occur exclusively
in the setting of delirium |
Once the presence of dementia is established
an attempt should be made to identify its etiology by use
of the history, clinical exam, neuropsychological assessment,
and, where feasible, imaging and laboratory studies. None
of the currently available biological markers are useful for
establishing with certainty the diagnosis of any of the most
common forms of dementia: Alzheimer's disease (AD), vascular
dementia (VaD), dementia with Lewy bodies (DLB) or frontotemporal
dementia (FTD) (13). Therefore, the clinician must rely on
clinical criteria for making these diagnoses.
Alzheimer's disease
Alzheimer's disease is the most common form of late-onset
dementia. The National Institute of Neurological and Communicative
Disorders and Stroke- AD and Related Disorders Association
(NINCDS-ADRDA) criteria for AD have been shown to have adequate
sensitivity and specificity (see Table 3). Patients nearly
always present with the primary complaint of memory difficulty,
articulated either by the patient or by the family. This is
frequently associated with visuospatial disorientation or
language dysfunction. These may manifest as a tendency to
get lost in familiar locations, reduction in the conceptual
precision of speech or impaired comprehension of complex linguistic
material. Occasionally patients with the neuropathologic changes
of AD present clinically with disruption of a single cognitive
domain other than memory, such as loss of visuospatial or
frontal-executive function or aphasia. Such patients may be
diagnosed with posterior cortical atrophy, frontal variant-AD
or aphasia-predominant AD (15). The diagnosis of AD should
be held in question if there is evidence that the patient's
cognition is being impacted by another psychiatric, systemic
or central nervous system disease. Thus, in patients with
depression, severe hypothyroidism or cerebrovascular disease
the diagnosis of possible rather than probable AD is appropriate
until the cause of the disease is evident.
Table 3 NINCDS-ADRDA Criteria for
the Diagnosis of Alzheimer's Disease (15)
|
I. Probable AD: Core Diagnostic Features
A. Dementia established by clinical examination
(including MMSE, BRDRS, and neuropsychological testing)
B. Deficit in at least two areas of cognition
i. Memory (required)
ii. Other area besides memory
C. Deficits characterized by gradual onset and
progression, onset after age 40
D. Other systemic disorders or brain disease
do not account for the progressive deficits in memory
and cognition in and of themselves
II. Possible AD: Core Diagnostic Features
A. Dementia syndrome in the absence
of other neurologic, psychiatric, or systemic disorder,
OR
B. Presence of a second systemic or brain disorder
sufficient to produce dementia, which is not considered
to be the primary cause of the dementia
III. Features that make a diagnosis
of Probable or Possible AD unlikely or uncertain
A. Sudden apoplectic onset
B. Focal neurologic findings such as hemiparesis,
sensory loss, visual field deficits, and incoordination
early in the course of the illness
C. Seizures or gait disturbances at the onset
or very early in the course of the illness
IV. Criteria for diagnosis of Definite
Alzheimer's disease:
A. Clinical criteria for probable Alzheimer's disease
B. Histopathologic evidence obtained
from a biopsy or autopsy
As AD progresses, patients frequently
suffer from neuropsychiatric complications such as agitation,
apathy, delusions, hallucinations or depression. In
many cases, these constitute a greater burden for caregivers
than the cognitive deficits and may require treatment
with psychoactive medications.
|
Vascular dementia
Dementia due to cerebrovascular disease should be suspected
when impairment in more than one cognitive domain accompanies
clinical or neuroimaging evidence of stroke. Observed loss
of normal social and occupational functioning required in
the definition of dementia should result from cognitive impairment
and not be explained entirely by the physical disability that
results from stroke. The diagnosis is more certain when there
is a temporal association between clinical stroke and onset
of cognitive impairment, or when family members describe a
stepwise pattern of deterioration. The most common type of
VaD is associated with ischemic injury to subcortical white
matter and lacunar infarctions secondary to small vessel disease.
Patients with VaD often exhibit cortical deficits
associated with focal cerebral lesions, such as aphasia, neglect,
apraxia or dyscalculia. Frontal executive function is commonly
impaired and memory defects follow the frontal-subcortical
pattern, in which patients encode memories adequately but
have difficulty retrieving them.
Four sets of criteria exist for the diagnosis
of VaD. None have been shown to have good specificity, but
all are sensitive. Of these, the Hachinski Ischemic Score
(Table 4) may identify the greatest number of patients with
VaD in spite of not including neuroimaging criteria (13,17).
A score of = 4 is suggestive of AD or other non-vascular causes
of dementia, while a score of = 7 is supportive of a diagnosis
of VaD.
Table 4 Hachinski Ischemic Score (16)
| Abrupt onset |
2 |
| Stepwise deterioration |
1 |
| Fluctuating course |
2 |
| Nocturnal confusion |
1 |
| Preservation of personality |
1 |
| Depression |
1 |
| Somatic complaints |
1 |
| Emotional incontinence |
1 |
| Hypertension |
1 |
| History of stroke |
2 |
| Associated atherosclerosis |
1 |
| Focal neurologic symptoms |
2 |
| Focal neurologic signs |
2 |
Dementia with Lewy Bodies
Dementia with Lewy bodies (DLB) has been defined clinically
as a dementia syndrome with parkinsonism, delusions, hallucinations
(especially visual), fluctuating alertness and sensitivity
to neuroleptic medications (See Table 5) (18). The criteria
have poor sensitivity but are very specific (19). The cognitive
profile is remarkable for deficits in attention, visuospatial
reasoning and frontal-subcortical function. When patients
with DLB are compared to patients with AD, memory is significantly
worse in AD, while visuospatial function and executive abilities
are worse in DLB (20). The core clinical features that may
be present include fluctuating cognition, visual hallucinations
and parkinsonism. Depression and rapid eye movement (REM)
sleep behavior disorder are also common in DLB.
Parkinson's disease (PD) is characterized by
rigidity, bradykinesia, rest tremor, loss of righting reflexes,
and beneficial response to dopaminergic therapy. Approximately
40% of patients with idiopathic PD meet criteria for dementia
(21). This usually follows a frontal-subcortical pattern,
in which frontal executive functions and memory retrieval
are the most impaired. Patients with PD and dementia typically
have Lewy bodies in the cortex at autopsy. The dementia of
PD may represent a variant of DLB.
Table 5 Criteria for Dementia with
Lewy Bodies (17)
|
I. Progressive cognitive decline interfering
with social and occupational functioning, usually including
deficits of attention, frontal subcortical skills and
visuospatial ability; memory impairment tends to be
a later finding
II. Two of the following core features
are necessary for the diagnosis of probable DLB, one
for the diagnosis of possible DLB:
A. Fluctuating cognition with pronounced variations
in attention and alertness
B. Recurrent visual hallucinations which are
typically well-formed and detailed
C. Spontaneous motor features of parkinsonism
III. Supportive features:
A. Repeated falls
B. Syncope
C. Transient loss of consciousness
D. Neuroleptic sensitivity
E. Systematized delusions
F. Hallucinations in other modalities
IV. A diagnosis of DLB is less likely
in the presence of:
A. Clinical or neuroimaging evidence of stroke
Clinical, laboratory or neuroimaging evidence
for other physical illness or brain disorder that accounts
for the clinical picture
|
Frontotemporal Dementia
Frontotemporal dementia (FTD) features early behavioral changes
preceding loss of memory, perception, spatial skills or praxis
(22). This disorder is less common than AD, VaD or DLB. As
is the case with other neurodegenerative diseases, the onset
is insidious and progressive. Those close to the patient frequently
notice a change in personality characterized by tactlessness,
disinhibition, poor impulse control, poor grooming and hygiene,
emotional blunting, mental rigidity and ritualized behaviors
(22). Some patients demonstrate hyperorality, which may manifest
as a craving for sweets, but patients have been described
who chewed compulsively on nonfood objects. Language is often
impacted, and may be characterized by stereotypies and echolalia.
Anomia and reduced verbal output are common. Snout, grasp
and palmomental reflexes may be present (see Table 6.) Onset
of the disorder is typically between the ages of 45 and 65
years.
The clinical syndromes of progressive nonfluent
aphasia and semantic dementia are most often associated with
the neuropathologic changes of FTD. The former is typically
manifested as the insidious onset of anomia that progresses
to nonfluent aphasia, while the latter is characterized by
early loss of word meaning that manifests as failure of single-word
production and comprehension (22). Patients with semantic
dementia often lose conceptual knowledge in other spheres,
resulting in prosopagnosia or visual agnosia (23). Primary
progressive aphasia often leads to complete or nearly complete
mutism.
Table 6 Criteria for Frontotemporal
Lobar Degeneration (21)
|
I. Core diagnostic features
A. Insidious onset and gradual
progression
B. Early decline in social interpersonal conduct
C. Early impairment in regulation of personal
conduct
D. Early emotional bluntingE. Early loss of insight
II. Supportive diagnostic features
A. Decline in personal hygiene
and grooming
1. Mental rigidity and inflexibility
2. Distractibility and impersistence
3. Hyperorality and dietary changes
4. Perseverative and stereotyped behavior
5. Utilization behavior
B. Speech and language
1. Altered speech output
- a. Aspontaneity and economy of speech
- b. Press of speech
2. Stereotypy of speech
3. Echolalia
4. Perseveration
5. Mutism
C. Physical signs
1. Primitive reflexes
2. Incontinence
3. Akinesia, rigidity and tremor
4. Low and labile blood pressure
D. Investigations
1. Neuropsychology: significant impairment of
frontal lobe tests in the absence of severe amnesia,
aphasia, or perceptuospatial disorder
2. Electroencephalography: normal on conventional
EEG despite clinically evident dementia
Brain imaging (structural and/or functional):
predominant frontal and/or anterior temporal abnormality
|
Treatment of Alzheimer's Disease
A great deal of research has focused on identifying
medications capable of slowing or delaying the progression
of AD. A placebo-controlled, double-blind study comparing
selegiline, vitamin E (alpha-tocopherol) and a combination
of both drugs demonstrated that all three treatments delayed
the onset of functional dependence and the need for institutionalization
compared to placebo (24). Combination of the two drugs did
not offer significant benefit over either drug alone. Since
vitamin E is inexpensive and relatively safe in patients who
are not on anticoagulation, it is now the standard of care
to administer 1000 IU twice daily to patients diagnosed with
AD. The value of vitamin E in ameliorating other forms of
dementia is not known.
Four medications that block the action of acetylcholinesterase
have been proven to be beneficial for AD. The first of these
to be approved, tacrine, is associated with liver toxicity
and requires QID dosing. Newer agents are less toxic and easier
to use, and tacrine is no longer frequently prescribed (25,26).
Donepezil is a cholinesterase inhibitor that does not require
monitoring of liver function tests and is dosed once per day.
The starting dose of 5 mg is therapeutic; many patients benefit
from titration to 10 mg. Rivastigmine is a cholinesterase
inhibitor that is dosed twice daily, starting with 1.5 mg
tablets. The dose can be increased at 4-week intervals to
3 mg BID, then 4.5 mg BID and finally 6 mg BID if desired
(27). Gastrointestinal side effects (such as nausea or weight
loss) have been reported in 15 to 45% of subjects and result
in discontinuation of the drug in up to 25% (25). Galantamine
is a cholinesterase inhibitor with comparable cognitive benefits
(28). The optimal dose identified is 16 to 24 mg/d, divided
into two daily doses. Galantamine typically is titrated from
4 mg BID, to 8 mg BID, and finally to 12mg BID. Cholinesterase
inhibitors have been shown to improve cognition and global
function compared to placebo. Improved behavior, delayed decline
in function, decreased caregiver burden and deferral of institutionalization
have been suggested by some studies (28,29). Cholinesterase
inhibitors may be useful in other dementias with cholinergic
deficits including the dementia of Parkinson's disease and
DLB (29).
Another approach to treating AD pharmacologically
is to prevent excitotoxicity by blocking N-methyl-D-aspartate
(NMDA) receptors in the brain. This is the rationale for the
use of memantine, which delays the onset of severe functional
disability in patients with AD (30), even in patients who
are already receiving donepezil (32). The medication is started
at 5 mg once per day is titrated weekly in increments of 5
mg, with a target dose of 10 mg BID after four weeks. From
a neuropsychiatric standpoint, the drug seems to reduce agitation
(31-33). Although memantine fares well against placebo in
terms of side effects, it may be associated rarely with confusion
or headaches (32). Table 7 summarizes the medications commonly
used in the treatment of AD.
The benefits of memantine and cholinesterase
inhibitors are modest, and new approaches will be important
for the prevention or postponement of AD. Amyloid accumulation
in the cortex is considered the primary lesion of AD and research
currently is focused on preventing amyloid aggregation. Trials
of a vaccine against amyloid have been disappointing thus
far, due to the development of encephalopathy in some patients
(34), but efforts will continue to focus on vaccination strategies
as well as on beta and gamma secretase inhibitors, copper
and zinc chelators, statins, antioxidants and non-steroidal
anti-inflammatory drugs as means of limiting amyloid or amyloid-related
neuronal injury.
Most patients will develop neuropsychiatric
symptoms during the course of the illness. These symptoms
constitute a weighty burden on caregivers who should be advised
that modification of the patient's environment may reduce
the frequency and severity of these symptoms. Such modifications
may include avoiding overstimulation, following a regular
schedule, and keeping the patient active during the day but
providing quiet relaxing evenings. Cholinesterase inhibitors
and memantine have behavioral as well as cognitive benefits.
Many patients, however, will require psychotropic medications
for neuropsychiatric symptoms. Depression is a feature that
commonly accompanies AD and responds most readily to a non-sedating
serotonin-selective reuptake inhibitor (SSRI), such as sertraline
or escitalopram. Tricyclic antidepressants are of limited
usefulness because of sedation and anticholinergic side effects.
Agitation is a common complaint that may be associated with
depression, delusions, hallucinations or insomnia. Depending
on the associated features, clinicians may find use of an
atypical antipsychotic, antidepressant or anticonvulsant to
be useful for reducing agitation. In one trial for agitation
in dementia, risperidone was shown to be as effective as haloperidol,
with fewer extrapyramidal side effects (36). The effective
dose of risperidone is typically 1.0 to 1.5 mg/day. Low-dose
olanzapine (5-10 mg) reduced psychosis and agitation in an
18-week study of patients with possible or probable AD, with
no significant increase in extrapyramidal side effects (37).
Quetiapine represents a feasible alternative to these agents
and may produce fewer extrapyramidal side effects. One preliminary
study of sertraline use for agitation and aggression in severe
AD indicated that some patients responded favorably (38).
Trazodone is an unconventional antidepressant with hypnotic
properties that is useful for insomnia and intermittent agitation
in demented patients (21). In some cases, neuropsychiatric
symptoms may respond to an anticonvulsant with mood stabilizing
properties (39,40).
The late stages of AD and most dementias are
marked by complete functional dependence. Patients are non-ambulatory,
unable to communicate needs and may be unable to feed themselves.
End of life issues must be addressed with patients and family
members before this stage is reached, as many people have
strong feelings regarding use of intravenous hydration or
nasogastric or percutaneous gastrostomy tubes for life support.
As with all bedridden patients, there is high risk for the
development of decubitus ulcers, dehydration, urinary tract
infection, pneumonia and deep venous thrombosis. These risks
may be reduced by adequate physical therapy, frequent turning,
and hydration. Encephalopathy, rather than fever, is often
the earliest sign of infection and should warrant prompt evaluation,
as infections are frequently the cause of death in patients
with dementia.
The Caregiver Alliance
Those caring for demented patients bear a great
physical and emotional burden. Studies of medications for
AD have begun to take this into account; for example, use
of memantine is associated with a reduction in the caregiver
time requirement of about 45.8 hours per month (31). Regardless
of such modest improvements, caregivers remain responsible
for numerous time-consuming tasks, such as supervising patients
in activities of daily living, administering medications,
and restricting driving. In addition, the difficulty of caring
for a patient with dementia has a negative impact on the health
of the caregiver (41). As dementia becomes more prevalent
in society, resources that mitigate the suffering of caregivers
and assist them in coping with the daily management of the
patient will become increasingly necessary.
Table 7 Medications used in the treatment
of AD
Cognitive Agents
|
Starting Dose |
Target Dose |
Uses |
| Donepezil |
5 mg daily
|
10 mg daily |
Improve cognition, may reduce apathy and
hallucinations |
| Galantamine |
4 mg BID
|
12 mg BID |
|
| Rivastigmine |
1.5 mg BID
|
6 mg BID |
|
| Memantine |
5 mg daily
|
10 mg BID |
Slow functional decline, may improve agitation |
| Antidepressants |
|
|
|
| Sertraline |
25 mg daily
|
75-100 mg daily |
Depression and agitation |
Escitalopram
|
5 mg daily
|
10-20 mg daily |
Depression |
| Trazodone |
25 mg QHS
|
100-400 mg daily |
Agitation, insomnia |
| Atypical Antipsychotics |
|
|
|
| Risperidone |
0.25 mg daily |
0.75-1.5 mg daily |
Agitation, delusions, hallucinations |
Conclusion
The prevalence of dementia is rising as the
aged segment of the population grows larger. This growth is
out of proportion to growth in the younger segments of the
population, and dementia will impose an increasing social
and economic burden during the next several decades. Middle-Eastern
nations will experience a marked growth in aged segments of
their populations in the impending decades. The diagnosis
of dementia is best made with clinical assessment including
cognitive testing. Treatments that delay the progression or
improve the symptoms of AD include cholinesterase inhibitors
and vitamin E. The management of neuropsychiatric symptoms
is important for improving quality of life for patients and
caregivers. Researchers and clinicians must recognize cognitive
decline early and identify treatments that will delay or prevent
the onset of dementia. Current trials are focused on identifying
reliable biological markers of dementia, preventing the advancement
of MCI to AD, and finding treatments to slow or halt the progression
of AD and other dementing diseases.

ACKNOWLEDGMENTS
Dr. Cummings receives support from an Alzheimer's
Disease Research Center grant (PSOAG16570) from the National
Institute on Aging, and Alzheimer's Disease Research Center
of California grant and the Sidell-Kagan Foundation. Dr. Clark
is supported by the Veteran's Affairs Special Fellowship,
Geriatric Neurology Section.
REFERENCES
| 1. |
Weintraub S. (2000). Neuropsychological
Assessment of Mental State. In Principles of Cognitive
and Behavioral Neurology, M.-Marsel Mesulam, ed. Oxford
University Press, New York, NY. |
| 2. |
Statistics available online
at http://devdata.worldbank.org/hnpstats/ |
| 3. |
Brookmeyer R, Gray S and Kawas
C. (1998). Projections of Alzheimer's disease in the United
States and the public health impact of delaying disease
onset. American Journal of Public Health 88: 1337-1342.
|
| 4. |
Petersen RC, Doody R, Kurz
A, Mohs RC, et al. (2001). Current concepts in mild cognitive
impairment. Archives of Neurology 58: 1985-1992. |
| 5. |
Petersen RC. (2003). Mild cognitive
impairment clinical trials. Nature Reviews Drug Discovery
2: 646-653. |
| 6. |
Petersen RC, Stevens JC, Ganguli
M et al. (2001). Practice parameter: Early detection of
dementia: Mild cognitive impairment (an evidence-based
review). Neurology 56: 1133-1142 |
| 7. |
Kukull WA, Larson, EB, Teri
L et al. (1994). The Mini-Mental Status Exam score and
the clinical diagnosis of dementia. Journal of Clinical
Epidemiology 47: 1061-1067. |
| 8. |
Al Rajeh S, Ogunniyi A, Awada
A, Daif AK, and Zaidan R. (1998). Validation of the Arabic
version of the mini-mental state examination. Annals of
Saudi Medicine 19(2): 150-152. |
| 9. |
Galasko D, Bennet D, Sano
M et al. (1997). An inventory to assess activities of
daily living for clinical trials in Alzheimer's disease.
Alzheimer Disease and Associated Disorders. 11: S33-S39. |
| 10. |
Morris JC. (1993). The Clinical
Dementia Rating (CDR): Current version and scoring rules.
Neurology 43: 2412-2414. |
| 11. |
Arya OP. (1996). Endemic treponematoses,
in Manson's Tropical Diseases, G. C. Cook, ed. W. B. Saunders:
London. |
| 12. |
Hsich G, Kenney K, Gibbs CJ,
Lee KH, Harrington MG. (1996). The 14-3-3 brain protein
in cerebrospinal fluid as a marker for transmissible spongiform
encephalopathies. New England Journal of Medicine 335:
924-930. |
| 13. |
Knopman DS, DeKosky ST, Cummings
JL et al. (2001). Practice parameter: Diagnosis of dementia
(an evidence-based review). Neurology 56: 1143-1153. |
| 14. |
American Psychiatric Association.
Diagnostic and Statistical Manual of Mental Disorders:
DSM-IV, 4th edition. Washington, DC: American Psychiatric
Association, 1994. |
| 15. |
Galton CJ, Patterson K, Xuereb
JH and Hodges JR. (2000). Atypical and typical presentations
of Alzheimer's disease: a clinical, neuropsychological,
neuroimaging and pathological study of 13 cases. Brain
123: 484-498. |
| 16. |
McKhann G, Drachman D, Folstein
M et al. (1984). Clinical diagnosis of Alzheimer's disease:
report of the NINCDS-ADRDA Work Group under the auspices
of the Department of Health and Human Services Task Force
on Alzheimer's disease. Neurology 34: 939-974. |
| 17. |
Moroney JT, Bagiella E, Desmond
DW et al. (1997). Meta-analysis of the Hachinski Ischemic
Score in pathologically verified dementias. Neurology
49: 1096-1105. |
| 18. |
McKeith IG, Galasko D, Kosaka
K et al. (1996). Consensus guidelines for the clinical
and pathologic diagnosis of dementia with Lewy bodies
(DLB): report of the consortium on DLB international workshop.
Neurology 47: 1113-1124. |
| 19. |
Holmes C, Cairns N, Lantos P and Mann
A. (1999). Validity of current clinical criteria for
Alzheimer's disease, vascular dementia and dementia
with Lewy bodies. British Journal of Psychiatry 174:
45-50.
|
| 20. |
Salmon DP, Galasko D, Hansen
LA et al. (1996). Neuropsychological deficits associated
with diffuse Lewy body disease. Brain and Cognition 31:
148-165. |
| 21. |
Cummings JL and Trimble MR.
(2002). Concise Guide to Neuropsychiatry and Behavioral
Neurology, 2nd ed. American Psychiatric Publishing, Washington,
D.C. |
| 22. |
Neary D, Snowden JS, Gustafson
L et al. (1998). Frontotemporal lobar degeneration: A
consensus on clinical diagnostic criteria. Neurology 51:
1546-1554. |
| 23. |
Hodges, JR. (2001). Frontotemporal
dementia (Pick's disease): clinical features and assessment.
Neurology 56(Suppl 4): S6-S10. |
| 24. |
Sano M, Ernesto C, Thomas RG
et al. (1997). A controlled trial of selegiline, alpha-tocopherol,
or both as treatment for Alzheimer's disease. New England
Journal of Medicine 336: 1216-1222. |
| 25. |
Doody RS, Stevens JC, Beck
C et al. (2001). Practice parameter: Management of dementia
(an evidence-based review). Neurology 56: 1154-1166. |
| 26. |
Rogers SL, Farlow, MR, Doody
RS, Mohs R, Friedhoff LT and the Donepezil Study Group.
(1998). A 24-week, double-blind, placebo-controlled trial
of donepezil in patients with Alzheimer's disease. Neurology
50: 136-145. |
| 27. |
Rösler M, Anand R, Cicin-Sain
A et al. (1999). Efficacy and safety of rivastigmine in
patients with Alzheimer's disease: international randomised
controlled trial. British Medical Journal 318: 633-640. |
| 28. |
Raskind MA, Peskind ER, Wessel
T, Yuan W and the Galantamine USA-1 Study Group. (2000).
Galantamine in AD. A 6-month randomized, placebo-controlled
trial with a 6-month extension. Neurology 54: 2261-2268. |
| 29. |
Cummings JL. (2000). Cholinesterase
inhibitors: a new class of psychotropic compounds. 157:
4-15. |
| 30. |
Mohs RC, Doody RS, Morris JC
et al. (2001). A 1-year, placebo-controlled preservation
of function survival study of donepezil in AD patients.
57: 481-488. |
| 31. |
Reisberg B, Doody R, Stoffler
A, Schmitt F, et al. (2003). Memantine in moderate-to-severe
Alzheimer's disease. The New England Journal of Medicine
348(14): 1333-1341. |
| 32. |
Tarriot PN, Farlow MR, Grossberg
GT, Graham SM, et al. (2004). Memantine treatment in patients
with moderate to severe Alzheimer disease already receiving
donepezil. A randomized placebo controlled trial. Journal
of the American Medical Association 291: 317-324. |
| 33. |
Livingston G and Katona C.
(2004). The place of memantine in the treatment of Alzheimer's
disease: a number needed to treat analysis. International
Journal of Geriatric Psychiatry 19: 919-925. |
| 34. |
Dominguez DI and De Strooper
B. (2002). Novel therapeutic strategies provide the real
test for the amyloid hypothesis of Alzheimer's disease.
Trends in Pharmacological Sciences 23(7): 324-330. |
| 35. |
Feldman H, Gauthier S, Hecker
J et al. (2001). A 24-week, randomized, double-blind study
of donepezil in moderate to severe Alzheimer's disease.
Neurology 57: 613-620. |
| 36. |
Chan W, Lam LC, Choy CN, Leung
VP, Li S and Chiu HF. (2001). A double-blind randomized
comparison of risperidone and haloperidol in the treatment
of behavioral and psychological symptoms in Chinese dementia
patients. International Journal of Geriatric Psychiatry
16: 1156-1162. |
| 37. |
. Street JL, Clark WS, Kadam
DL et al. (2001). Long-term efficacy of olanzepine in
the control of psychotic and behavioral symptoms in nursing
home patients with Alzheimer's dementia. International
Journal of Geriatric Psychiatry 16: S62-S70. |
| 38. |
Lanctôt KL, Herrman N,
van Reekum R, Eryavec G and Naranjo CA. (2002). Gender,
aggression and serotonergic function are associated with
response to sertraline for behavioral disturbances in
Alzheimer's disease. International Journal of Geriatric
Psychiatry 17: 531-541. |
| 39. |
Grossman F. (1998). A review
of anticonvulsants in treating agitated demented elderly
patients. Pharmacotherapy 18(3): 600-606. |
| 40. |
Moretti R, Torre P, Antonello
M and Cazzato G. (2001). Gabapentin as a possible treatment
of behavioral alterations in Alzheimer disease (AD) patients
(letter). European Journal of Neurology 8: 501-502. |
| 41. |
Schultz R and Martire LM. (2004).
Family caregiving of persons with dementia. Prevalence,
health effects, and support strategies. American Journal
of Geriatric Psychiatry 12(3): 240-249. |
|


