|
|
 |
| ............................................................. |
|
|
| ........................................................ |
| From
the Editor |

|
Editorial
A. Abyad (Chief Editor) |
|
|
|
|
........................................................ |
Original
Contribution / Clinical Investigation
........................................................
International Health
Affairs
........................................................
Education
and Training
........................................................
Case
Study
|
Chief
Editor -
Abdulrazak
Abyad
MD, MPH, MBA, AGSF, AFCHSE
.........................................................
Editorial
Office -
Abyad Medical Center & Middle East Longevity
Institute
Azmi Street, Abdo Center,
PO BOX 618
Tripoli, Lebanon
Phone: (961) 6-443684
Fax: (961) 6-443685
Email:
aabyad@cyberia.net.lb
.........................................................
Publisher
-
Lesley
Pocock
medi+WORLD International
11 Colston Avenue,
Sherbrooke 3789
AUSTRALIA
Phone: +61 (3) 9005 9847
Fax: +61 (3) 9012 5857
Email:
lesleypocock@mediworld.com.au
.........................................................
Editorial
Enquiries -
abyad@cyberia.net.lb
.........................................................
Advertising
Enquiries -
lesleypocock@mediworld.com.au
.........................................................
While all
efforts have been made to ensure the accuracy
of the information in this journal, opinions
expressed are those of the authors and do not
necessarily reflect the views of The Publishers,
Editor or the Editorial Board. The publishers,
Editor and Editorial Board cannot be held responsible
for errors or any consequences arising from
the use of information contained in this journal;
or the views and opinions expressed. Publication
of any advertisements does not constitute any
endorsement by the Publishers and Editors of
the product advertised.
The contents
of this journal are copyright. Apart from any
fair dealing for purposes of private study,
research, criticism or review, as permitted
under the Australian Copyright Act, no part
of this program may be reproduced without the
permission of the publisher.
|
|
|
| January 2017 -
Volume 15, Issue 1 |
|
|
Concentrations of Azithromycin
and Amoxicillin-Clavulanic acid
in patients undergoing tonsillectomy
Nidhal AK
Mohammed Ali (1)
Rasha G. Thanoon (2)
(1)
Ph.D. Assistant Professor, Department
of Pharmacology, College of Medicine,
Hawler Medical University.
(2) MSc. Registration Department of Kurdistan
Medical Control Agency,
Ministry of Health, Kurdistan Region, Iraq
Correspondence:
Nidhal
AK Mohammed Ali
Department of Pharmacology
College of Medicine
Hawler Medical University
Tel: +9647504369240
Email: nidhal.mali@med.hmu.edu.iq
|
Abstract
Background: In treating microbial
infections, it is important to choose
an antibiotic with appropriate spectrum
of activity and one that achieves adequate
concentration for a sufficient period
of time at the site of infection. This
concept becomes necessary when antibiotics
fail to cure infection, along with increasing
emergence of antimicrobial resistance.
Aim: The aim of the study is to
assess the antibacterial activity of two
antimicrobial agents indicated in the
treatment of tonsillitis; azithromycin
and amoxicillin-clavulanic acid.
Methods: A single blind comparative
study was conducted on 43 patients with
recurrent tonsillitis with mean age of
5.46±2.38 years who were scheduled
for tonsillectomy in ENT department, Rizgary
Hospital. The patients were allocated
randomly into 2 groups. Group 1 patients
(n=20) were given azithromycin and group
2 patients (n=23) received amoxicillin-clavulanic
acid at the recommended dose for each
antibiotic. Bacterial isolation and identification
were performed and minimum inhibitory
concentrations (MIC) of isolated bacteria
were determined. Blood and tonsillar tissue
samples were taken from each patient before,
and 2 hours after, drug administration.
The plasma and tonsillar tissue concentration
of each antibiotic were determined.
Results: Staphylococcus aureus
was the most predominant organism isolated
from the patients. Azithromycin and amoxicillin-clavulanic
acid attained mean plasma concentration
of 0.27±0.04µg/ml
and 5.49±0.33µg/ml respectively
and the mean azithromycin concentration
in tonsils tissues was 13.97± 2.75µg/g
whereas no detectable concentrations of
amoxicillin-clavulanic acid were determined
in the tonsils tissue of the patients.
Conclusion: Azithromycin achieved
higher tissue concentration than amoxicillin-clavulanic
acid in tonsils tissues making this antibiotic
a good choice for recurrent tonsillitis.
Key words: azithromycin, amoxicillin-clavulanic
acid, tonsillitis, pharyngitis, resistance.
|
There are continuous reports relating to failure
of antimicrobial therapy to emergence of bacterial
resistance (1, 2). This resistance problem markedly
encouraged reassessment of antibacterial effectiveness
of microbial infections with resistant organisms
(3, 4).
Although, Streptococci group A beta-hemolytic
(GABHS) is the main cause of pharyngo-tonsillitis
(5), other bacteria such as S. aureus, S. pneumoniae,
H. influenzae are also isolated (6,7). . Penicillin
V is considered the drug of choice for the treatment
of GABHS pharyngitis however it is not effective
when the infection is caused by beta-lactamase-producing
bacteria (8). Amoxicillin is an amino-penicillin
with extended spectrum of activity combined
with clavulanic acid (Amoxiclav)® to broaden
its activity against resistant organisms (8).
The semi-synthetic macrolide, azithromycin is
effective against a wide variety of bacteria
including those causing pharyngo-tonsilitis
and is usually reserved for patients who are
allergic to Penicillins (8, 9).
Although these antibiotics possess broad spectrum
activity that cover most pathogens causing pharyngitis,
they are still unsuccessful in preventing recurrences
of these infections and 7%-37% of children treated
with an appropriate antibiotic are considered
bacteriologic failures (9, 10). This problem
could be related to either infection with resistant
bacteria or failure of drugs to achieve adequate
antimicrobial concentrations in the site of
infection (2). Therefore, this study was designed
to compare the effectiveness of two commercially
available antimicrobial agents indicated in
the treatment of tonsillitis; azithromycin and
amoxicillin/clavulanate by estimating their
concentrations in plasma and tonsils tissue
of children undergoing tonsillectomy and relate
these levels with the minimal inhibitory concentrations
(MIC) of the bacteria isolated from the patient's
tonsils.
The
study
design
was
a
single
blind
comparative
study
that
included
forty
three
children
aged
between
2-14
years
of
both
gender
with
recurrent
tonsillitis
who
were
scheduled
for
tonsillectomy
with
no
history
of
allergy
to
beta-lactams
or
macrolides
antibiotics.
Patients
with
preexisting
medical
condition
that
might
affect
drug
pharmacokinetics
or
requiring
perioperative
antibiotics
(i.e.,
endocarditis),
or
with
history
of
antibiotic
use
within
2
weeks
prior
to
tonsillectomy
or
with
history
of
significant
hematological,
renal
and
hepatic
disease,
were
excluded
from
the
study.
The
study
was
conducted
with
the
approval
of
the
Ethical
Committee
of
the
College
of
Medicine,
Hawler
Medical
University
and
informed
consent
was
taken
from
parents
of
each
patient
after
explaining
the
study
protocol
in
keeping
with
the
Ethical
Committee
policy.
The
patients
were
allocated
randomly
into
2
groups.
The
children
were
given
the
drug
suspension
by
a
calibrated
syringe
so
that
the
volume
of
suspension
given
is
measured
precisely.
Group
1
patients
(n=23)
were
given
amoxicillin-clavulanic
acid
(Julmentin®;
Julphar,
UAE)
and
azithromycin
(Zomzx®;
Hikma,
Jordan)
was
given
to
group
2
patients
(n=20).
The
drugs
were
given
orally
a
day
before
and
approximately
2
hours
before
the
scheduled
time
of
surgery
at
the
recommended
dose
of
10
mg/kg
for
azithromycin
and
156mg/5ml
(24.96
mg/kg/day)
for
amoxicillin-clavulanic
acid.
Before
starting
medications,
sterile
swabs
were
taken
from
the
core
of
the
tonsil
of
each
patient
for
microbiological
isolation
of
bacteria
(11)
and
thereafter
bacteria
were
identified
to
the
species
level
by
VITEK
2
colorimetric
identification
card
(12).
The
minimum
inhibitory
concentration
(MIC)
of
each
isolate
was
determined
by
broth
dilution
method
according
to
the
National
Committee
for
Clinical
Laboratory
Standards
(13).
Samples
from
venous
blood
were
taken
from
each
patient
before
drug
administration
and
at
time
of
operation
corresponding
to
2
hours
after
drug
administration.
The
blood
samples
were
collected
in
heparinized
tubes
and
plasma
was
obtained
by
centrifugation
of
blood
samples
for
10
minutes.
Tonsils
were
taken
at
the
time
of
operation
at
the
surgical
theatre
at
times
relevant
to
timing
of
the
blood
samples,
weighed,
wiped
gently
with
dry
sterile
gauze.
Plasma
and
tonsils
samples
were
immediately
stored
in
deep
freeze
(-40
C)
until
analyzed
by
the
microbiological
assay
method
using
standard
S.
aureus
ATCC
(6538P)
sensitive
to
azithromycin
and
amoxicillin-clavulanic
acid
according
to
(14,
15)
respectively.
For
the
determination
of
drugs
concentrations,
drug-free
plasma
and
tonsil
samples
were
spiked
with
different
concentrations
of
each
drug
separately.
The
standard
concentrations
were
analyzed
in
triplicate
along
with
the
samples
by
the
microbiological
assay
method
mentioned
above
and
a
standard
curve
was
generated
relating
the
diameter
of
zone
of
inhibition
(mm)
with
different
concentrations
of
either
drug.
Calculations
of
azithromycin
and
amoxicillin-clavulanic
acid
concentrations
in
plasma
and
tonsils
samples
were
determined
according
to
(16).
The
limit
of
detection
for
azithromycin
and
amoxicillin
-clavulanic
acid
in
plasma
and
tonsils
were
0.01
µ
g/ml
and
(0.05
µg/g
respectively.
SPSS
version
19
was
used
to
analyze
the
differences
between
different
concentrations
of
the
drugs
in
plasma
and
tonsils
samples.
A
P
<
0.05
was
considered
statistically
significant
difference.
The
mean
age,
weight
and
distribution
of
gender
of
patients
enrolled
in
the
study
in
both
treatment
groups
are
shown
in
Table
1.
Table
1:
Demographic
characteristics
of
patients
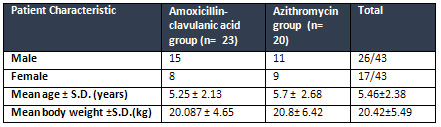
The
mean
age
of
children
was
5.25±2.13
years
and
5.7
±
2.68
year
for
amoxicillin-clavulanic
acid
and
azithromycin
group
respectively
(Table
1).
The
children
had
a
mean
weight
of
20.42
±5.49
kg
and
the
ratio
of
distribution
of
male:
female
was
1.53:1
(Table
1).
Different
microorganisms
were
isolated
from
the
tonsils
taken
from
the
patients
and
S.
aureus
was
isolated
from
the
majority
of
the
patients
(Table
2).
Table
2:
Microorganisms
isolated
from
the
tonsils
of
patients
in
different
treatment
groups
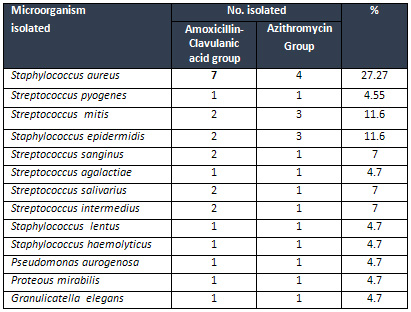
S.
pyogenes
isolated
from
patients
was
resistant
to
both
amoxicillin-clavulanic
acid
and
azithromycin
antimicrobial
agents
whereas
S.
agalactiae
was
sensitive
to
amoxicillin-clavulanic
acid
but
resistant
to
azithromycin.
Four
S.
aureus
isolates
were
sensitive,
2
were
intermediately
sensitive
and
only
one
was
resistant
to
amoxicillin-Clavulanic
acid
while
all
four
isolates
of
S.
aureus
were
resistant
to
azithromycin
antimicrobial
agent
as
shown
in
Table
(3).
Table
3:
Susceptibilities
of
different
micro-organisms
isolated
from
tonsils
of
the
patients
to
amoxicillin-clavulanic
acid
and
azithromycin
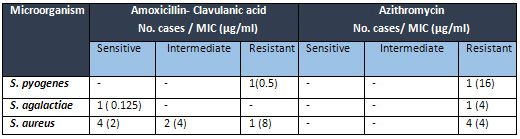
The
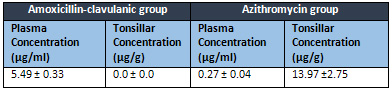
mean
concentrations
of
amoxicillin-clavulanic
acid
and
azithromycin
in
plasma
were
5.49±
0.33µg/ml
and
0.27
±
0.04µg/ml
respectively.
In
the
tonsils,
the
mean
concentrations
of
azithromycin
was
13.97
±
2.75
µg/g
whereas
no
detectable
concentrations
were
determined
for
amoxicillin-clavulanic
acid
in
tonsils
according
to
the
limit
of
detection
of
the
assay
(Table
4).
Table
4:
The
mean
concentration
of
Amoxicillin-clavulanic
group
and
Azithromycin
in
plasma
and
tonsils
tissue
To
obtain
an
effective
clinical
response
to
antimicrobial
therapy,
the
drug
should
reach
effective
concentration
at
the
site
of
infection
greater
than
the
MIC
of
the
causative
organisms
(3,
4).
S.
aureus
was
the
most
predominant
organism
(27.27%)
isolated
from
the
patients
in
the
present
study
and
has
also
been
reported
in
other
studies
as
the
most
predominant
pathogen
isolated
from
children
with
recurrent
tonsillitis
(6,
11).
S.
pyogenes
was
only
isolated
from
2
patients
(4.55%)
in
the
present
study,
which
is
also
in
accordance
to
other
findings
(11,
17).
Another
study
stated
that
among
a
total
of
294
children
with
acute
tonsillitis,
Group
A
streptococci
was
isolated
only
from
three
children
(18).
The
mean
plasma
amoxicillin
concentration
(5.49±0.33
µg/ml)
estimated
in
the
plasma
of
the
patients,
indicates
that
amoxicillin
is
well
absorbed
into
systemic
circulation.
Indeed
amoxicillin
has
high
oral
bioavailability
(70-90%)
with
peak
plasma
levels
occurring
within
1
to
2
hours
(19-21).
This
mean
plasma
levels
(5.49
µ
g/ml)
is
close
to
those
determined
(4.7
µ
g/ml)
in
children
(22)
although
higher
peak
concentration
of
7.32
µg/ml
and
10.8
µg/ml
was
detected
2
hours
in
adult
volunteers
given
amoxicillin-clavulanic
acid
at
a
dose
similar
to
those
administered
in
the
present
study
(20,21).
The
differences
in
the
concentrations
is
most
likely
related
to
differences
in
the
drug
formulations,
amount
of
dose
given
or
to
the
analytical
method
of
analysis.
Based
on
recommendations
of
therapeutic
effectiveness
of
beta-lactams
in
humans
and
experimental
studies;
the
concentrations
of
antibiotic
is
required
to
be
four
to
five
times
the
MIC
or
higher
when
associated
with
improved
outcome
especially
with
resistant
strains
(23,24)
thus,
although
levels
of
amoxicillin-clavulanic
acid
in
plasma
were
higher
than
the
MICs
of
the
isolated
species,
this
antibiotic
did
not
attain
effective
concentrations
in
the
site
of
infection
(tonsils)
to
eradicate
the
resistant
organisms
(
24).
The
S.
pyogenes
that
was
isolated
from
the
patients
was
considered
resistant
according
to
the
breakpoint
therefore
this
antibacterial
agent
would
not
provide
an
effective
treatment
especially
in
recurrent
cases
with
resistant
organisms.
Furthermore,
although
plasma
concentrations
are
generally
a
good
indicator
of
drug
effectiveness
they
are
usually
a
poor
indicator
of
intracellular
concentrations,
which
is
of
major
importance
for
intracellular
pathogens
including
S.
aureus
as
well
as
S.
pyogenes
that
are
also
shown
to
be
intracellular
pathogen
of
tonsils
(25,
26).
One
of
the
properties
that
characterize
beta-lactams
including
amoxicillin
is
that
they
are
weak
acids
and
quickly
diffuse
into
cells
and
because
the
cell
cytosol
is
more
acidic
than
extracellular
milieu
they
will
be
more
in
unionized
form
so
they
are
prevented
from
accumulating
in
the
cells
and
will
readily
be
absorbed
to
systemic
circulation
(27,
28).
Studies
recommended
administering
amoxicillin-clavulanic
acid
at
higher
dose
are
based
on
their
finding
that
one
high
dose
of
amoxicillin-clavulanic
acid
would
inhibit
the
biofilm
formed
in
the
tonsillar
tissue
therefore
exposing
the
bacteria
to
effective
treatment
since
recurrent
pharyno-tonsillitis
and
failure
of
treatment
has
been
attributed
to
biofilm
formation
(29,
30).
Concerning
azithromycin,
the
mean
plasma
concentrations
of
(0.27
±
0.04
µg/ml)
is
close
to
those
reported
(0.24
?g/ml)
in
children
receiving
30
mg
azithromycin
(31).
However,
lower
concentrations
in
plasma
(0.18
µ
g/ml)
were
estimated
by
(32)
and
0.13
µg/ml
by
(33).
These
differences
are
related
to
differences
in
dosing,
different
drug
formulations
and
method
of
drug
analysis.
The
mean
azithromycin
concentrations
(13.96
±
2.75
µg/g)
in
tonsillar
tissues
indicate
this
drug
undergoes
rapid
uptake
from
systemic
circulation
into
the
infection
site
thus
exposing
the
local
compartment
(tonsils)
to
azithromycin
concentrations
higher
than
plasma
levels.
High
ratio
of
azithromycin
concentration
in
tonsils
to
that
in
plasma
was
also
reported
and
shown
to
be
greater
than
plasma
concentrations
by
many
fold
for
all
time
intervals
after
administration
(15,
33).
One
of
the
remarkable
features
of
azithromycin
is
its
ability
to
accumulate
in
intracellular
compartments,
mainly
in
fibroblasts,
phagocytic
cells,
and
other
white
blood
cells
(34).
This
prominent
character
is
explained
by
its
dibasic
molecular
structure
that
allows
azithromycin
to
be
concentrated
within
the
acidic
lysosomes
of
white
blood
cells
due
to
an
ion-trapping
mechanism
from
where
it
will
be
released
very
slowly
from
intracellular
compartments
(35).
This
characteristic
is
believed
to
account
for
the
prolonged
drug
concentrations
in
tissues
which
are
reported
to
persist
long
after
the
end
of
therapy
and
reflected
by
a
long
elimination
half-life
of
up
to
5
days
(36
).
In
conclusion;
although
amoxicillin-clavulanic
acid
is
clinically
considered
more
effective
against
beta-lactamase
producing
organisms
and
is
the
most
frequently
prescribed
antibiotic
for
such
infection
(9,37)
azithromycin's
good
tissue
penetration,
once
daily
administration,
besides
its
immunomodulatory
effects
provides
further
benefits
along
with
its
dual
antibacterial
mode
of
action
(38,
39)
and
makes
this
antibiotic
a
good
choice
when
the
standard
penicillin
V
therapy
of
tonsilo-pharyngitis
fails.
Furthermore,
the
consequences
of
the
low
amoxicillin-clavulanic
acid
levels
in
tonsils
might
lead
to
potentially
negative
effects
on
clinical
response
and
emergence
of
resistances
(40).
1-
Huttner
A,
Harbarth
S,
Carlet
J,
Cosgrove
S,
Goossens
H,
Holmes
A,
et
al.
Antimicrobial
resistance:
a
global
view
from
the
2013
World
Healthcare-Associated
Infections
Forum.
Antimicrob
Resist
Infect
Control.
2013;
2:31-
41.
2-
Llor
C,
Bjerrum
L.
Antimicrobial
resistance:
risk
associated
with
antibiotic
overuse
and
initiatives
to
reduce
the
problem.
Ther
Adv
Drug
Saf.
2014;
5(6):
229-
41.
3-
Thabit
AK,
Crandon
JL,
Nicolau
DP.
Antimicrobial
resistance:
impact
on
clinical
and
economic
outcomes
and
the
need
for
new
antimicrobials.
Expert
Opin
Pharmacother.
2015;
16(2):159-77.
4-
Ventola
CL.
The
antibiotic
resistance
crisis:
part
1:
causes
and
threats.
P
T.
2015;
40(4):277-83.
5-
Michael
R.
Wessels,
M.D.
Streptococcal
Pharyngitis.
N
Engl
J
Med,
2011;
364:648-55.
6-
Zautner
AE,
Krause
M,
Stropahl
G,
Holtfreter
S,
Frickmann
H,
Maletzki
C,
et
al.
Intracellular
Persisting
Staphylococcus
aureus
is
the
Major
Pathogen
in
Recurrent
Tonsillitis.
PLos
One.
2010;
5(3):
e9452.
7-
Alasil
S,
Omar
R,
Ismail
S,
Yusof
MY,
Ameen
M.
Bacterial
identification
and
antibiotic
susceptibility
patterns
of
Staphyloccocus
aureus
isolates
from
patients
undergoing
tonsillectomy
in
Malaysian
University
Hospital.
Afr
J
Microb
Res.
2011;
5(27):
4748-52.
8-
Shulman
ST,
Bisno
AL,
Clegg
HW,
Gerber
MA,
Kaplan
EL,
Lee
G,
et
al.
Clinical
practice
guideline
for
the
diagnosis
and
management
of
group
A
streptococcal
pharyngitis:
2012
update
by
the
Infectious
Diseases
Society
of
America.
Clin
Infect
Dis.
2012;
55(10):
1279-82.
9-
Regoli
M,
Chiappini
E,
Bonsignori
F,
Galli
L,
de
Martino
M.
Update
on
the
management
of
acute
pharyngitis
in
children.
Ital
J
Pediatr.
2011;
37:10-17.
10-
Aalbers
J,
O'Brien
KK,
Chan
WS,
Falk
GA,
Teljeur
C,
Dimitrov
BD,
Fahey
T.
Predicting
streptococcal
pharyngitis
in
adults
in
primary
care:
a
systematic
review
of
the
diagnostic
accuracy
of
symptoms
and
signs
and
validation
of
the
Centor
score.
BMC
Med.
2011;
9:67-78.
11-
Babaiwa
UF,
Onyeagwara
NC
Akerele
JO.
Bacterial
tonsillar
microbiota
and
antibiogram
in
recurrent
tonsillitis.
Biomedical
Research.
2013;
24
(3):
298-302.
12-
Wallet
F,
Loiez
C,
Renaux
E,
Lemaitre
N,
Courcol
RJ.
Performances
of
VITEK
2
colorimetric
cards
for
identification
of
gram-positive
and
gram-negative
bacteria.
J
Clin
Microbiol.
2005;
43(9):4402-06.
13-
Clinical
and
Laboratory
Standards
Institute.
Performance
Standards
for
Antimicrobial
Susceptibility
Testing;
Twenty-First
Informational
Supplement.
Wayne,
PA.
USA.
2012;
32
(3):
M100-S22.
14-
Davies
BE,
Boon
R,
Horton
R,
Reubi,FC,
C
E
Descoeudres
CE.
Pharmacokinetics
of
amoxycillin
and
clavulanic
acid
in
haemodialysis
patients
following
intravenous
administration
of
Augmentin.
Br.
J.
clin.
Pharmac.
1988;
26:
385-90.
15-
Blandizzi
C,
Malizia
T,
Batoni,
G,
Ghelardi
E,
Baschiera
F,
Paolo
Bruschini
P,
et
al.
Distribution
of
Azithromycin
in
Plasma
and
Tonsil
Tissue
after
Repeated
Oral
Administration
of
10
or
20
Milligrams
per
Kilogram
in
Pediatric
Patients.
Antimicrob
Agents
Chemother.
2002;
46(5):
1594-96.
16-
Jusko,
WJ.
Guidelines
for
collection
and
analysis
of
pharmacokinetic
data.
In:
Shargel
L,
Wu-Pong
S,
Yu
A,
editors.
Applied
Biopharmacetics
and
Pharmacokinetics,
New
York:
McGraw-Hill;
2005:
8-27.
17-
Devi
U,
Borah
PK,
Mahanta
J.
The
prevalence
and
antimicrobial
susceptibility
patterns
of
beta-hemolytic
streptococci
colonizing
the
throats
of
schoolchildren
in
Assam,
India.
J
Infect
Dev
Ctries
2011;
5(11):804-08.
18-
Hsieh
TH,
Chen
PY,
Huang
FL,
Wang
JD,
Wang
LC,
Lin
HK,
et
al.
Are
empiric
antibiotics
for
acute
exudative
tonsillitis
needed
in
children?
J
Microbiol
Immunol
Infect.
2011;
44:
328-32.
19-
Navarro
SA.
New
formulations
of
amoxicillin/clavulanic
acid:
a
pharmacokinetic
and
pharmacodynamic
review.
Clin
Pharmacokinet.
2005;
44(11):1097-115.
20-
Mostafavi
SA,
Dormiani
K,
Khazaie
Y.
Pharmacokinetics
of
Amoxicillin/clavulanic
acid
after
oral
administration
of
new
suspensions
formulation
in
human
volunteers.
International
J
Pharmacology.
2007;
3(3):
265-69.
21-
Kaur,
RAO
R,
Nanda
S.
Amoxicillin:
A
broad
spectrum
antibiotic.
Int
J
Pharm
Pharm
Sci.
2011;
3
(3):
30-37.
22-
Averono
G,
Vidali
M,
Olina
M,
Basile
M,
Bagnati
M,
Bellomo
G,
Aluffi
P.
Evaluation
of
amoxicillin
plasma
and
tissue
levels
in
pediatric
patients
undergoing
tonsillectomy.
Int
J
Pediatr
Otorhinolaryngol.
2010;
74(9):995-98.
23-
Andes
D,
Craig
WA.
In
vivo
activities
of
amoxicillin
and
amoxicillin-clavulanate
against
Streptococcus
pneumoniae:
application
to
breakpoint
determinations.
Antimicrob
Agents
Chemother.
1998;
42:
2375-79.
24-
Haeseker
M,
Havenith
T,
Stolk
L,
Neef
C,
Bruggeman
C,
Verbon
A.
Is
the
standard
dose
of
amoxicillin-clavulanic
acid
sufficient?
BMC
Pharmacol
Toxicol.
2014;
15:
38-46.
25-
Fraunholz
M,
Sinha
B.
Intracellular
Staphylococcus
aureus:
live-in
and
let
die.
Front
Cell
Infect
Microbiol.
2012;
2:43-
50.
26-
Fischetti
VA,
Dale
JB.
One
More
Disguise
in
the
Stealth
Behavior
of
Streptococcus
pyogenes.
mBio.
2016;
7(3):
e00661-16.
27-
Jensen
A,
Fago-Olsen,
H,
Sørensen
CH,
Kilian
M.
Molecular
Mapping
to
Species
Level
of
the
Tonsillar
Crypt
Microbiota
Associated
with
Health
and
Recurrent
Tonsillitis.
Plos
One.
2013;
8(2):
e56418.
28-
Yamanaka,
N.
Moving
towards
a
New
Era
in
the
Research
of
Tonsils
and
Mucosal
Barriers.
Adv
Otorhinolaryngol.
2011;
72:
6-19.
29-
Roberts
AL,
Connolly
KL,
Kirse
DJ,
Evans
AK,
Poehling
KA,
Peters
TR,
et
al.
Detection
of
group
A
Streptococcus
in
tonsils
from
pediatric
patients
reveals
high
rate
of
asymptomatic
streptococcal
carriage.
BMC
Pediatrics.
2012;
12:
3-11.
30-
Alasil
SM,
Omar
R,
Ismail
S,
Yusof
MY,
Dhabaan
GN,
Abdulla
MA.
Evidence
of
Bacterial
Biofilms
among
Infected
and
Hypertrophied
Tonsils
in
Correlation
with
the
Microbiology,
Histopathology,
and
Clinical
Symptoms
of
Tonsillar
Diseases.
Int
J
Otolaryngol.
2013;
2013:408238.
31-
Liu
P,
Fang,
AF,
LaBadie
RR,
Crownover
PH,
Arguedas
AG.
Comparison
of
Azithromycin
Pharmacokinetics
following
Single
Oral
Doses
of
Extended-Release
and
Immediate-Release
Formulations
in
Children
with
Acute
Otitis
Media.
Antimicrobial
agents
and
chemotherapy.
2011;
55(11):
5022-26.
32
Danesi
R,
Lupetti
A,
Barbara
C,
Ghelardi
E,
Chella
A,
Malizia
T,
et
al.
Comparative
distribution
of
azithromycin
in
lung
tissue
of
patients
given
oral
daily
doses
of
500
and
1000
mg.
J
Antimicrob
Chemother.
2003;
51(4):
939-45.
33-
Baschiera
F,
Fornai
M,
Lazzeri,
G,
Blandizzi
C,
Bruschinin
P,
Tacca
MD.
Improved
tonsillar
disposition
of
azithromycin
following
a
3-day
oral
treatment
with
20
mg
kg-1
in
paediatric
patients.
Pharmacol
Res.
2002;
46(1):
95-100.
34-
Amsden
GW.
Advanced-generation
macrolides:
tissue-directed
antibiotics.
Int
J
Antimicrob
Agents.
2001;
18
(S1):S11-15.
35-
Hand
WL,
Hand
DL.
Characteristics
and
mechanisms
of
azithromycin
accumulation
and
efflux
in
human
polymorphonuclear
leukocytes.
Int
J
Antimicrob
Agents.
2001;
18(5):419-25.
36-
Bosnar
M,
Kelneric
Z,
Munic
V,
Erakovic
V,
Parnham
MJ.
Cellular
uptake
and
efflux
of
azithromycin,
erythromycin,
clarithromycin,
telithromycin,
and
cethromycin.
Antimicrob
Agents
Chemother.
2005;
49(6):
2372-77.
37-
Mollahaliloglu
S,
Alkan
A,
Donertas
B,
Ozgulcu
S,
Akici
A.
Assessment
of
antibiotic
prescribing
at
different
hospitals
and
primary
health
care
facilities.
Saudi
Pharm
J.
2013;
21
(3):
281-91.
38-
Jelic
D,
Antolovic
R.
From
Erythromycin
to
Azithromycin
and
New
Potential
Ribosome-Binding
Antimicrobials.
Antibiotics.
2016;
5(3):
E29.
39-
Kanoh
S.
&
Rubin
BK.
Mechanisms
of
Action
and
Clinical
Application
of
Macrolides
as
Immunomodulatory
Medications.
Clin.
Microbiol.
Rev.
2010;
23,
590-615.
40-
Taccone
FS,
Laterre
PF,
Dugernier
T,
Spapen
H,
Delattre
I.
Insufficient
?-lactam
concentrations
in
the
early
phase
of
severe
sepsis
and
septic
shock.
Crit
Care.
2010;
14:R126.
|
|
.................................................................................................................

|
| |
|

