|
|
 |
| ............................................................. |
|
|
| ........................................................ |
| From
the Editor |

|
Editorial
A. Abyad (Chief Editor) |
|
|
|
|
........................................................
|
Original
Contribution/Clinical Investigation
|
|

|
<-- Turkey -->
Very high
levels of C-reactive protein should alert the
clinician to the development of acute chest
syndrome in sickle cell patients
[pdf version]
Can Acipayam, Sadik Kaya, Mehmet Rami Helvaci,
Gül Ilhan, Gönül Oktay
<-- Jordan -->
Seroprevalence
of HBV, HCV, HIV and syphilis infections among
blood donors at Blood Bank of King Hussein Medical
Center: A 3 Year Study
[pdf
version]
Baheieh Al Abaddi, Maha Al Amr, Lamees Abasi,
Abeer Saleem, Nisreen Abu hazeem, Ahmd Marafi
|
|
........................................................ |
Medicine and Society
........................................................
International Health
Affairs
.......................................................
Education
and Training
.......................................................
Continuing
Medical Education
|
Chief
Editor -
Abdulrazak
Abyad
MD, MPH, MBA, AGSF, AFCHSE
.........................................................
Editorial
Office -
Abyad Medical Center & Middle East Longevity
Institute
Azmi Street, Abdo Center,
PO BOX 618
Tripoli, Lebanon
Phone: (961) 6-443684
Fax: (961) 6-443685
Email:
aabyad@cyberia.net.lb
.........................................................
Publisher
-
Lesley
Pocock
medi+WORLD International
11 Colston Avenue,
Sherbrooke 3789
AUSTRALIA
Phone: +61 (3) 9005 9847
Fax: +61 (3) 9012 5857
Email:
lesleypocock@mediworld.com.au
.........................................................
Editorial
Enquiries -
abyad@cyberia.net.lb
.........................................................
Advertising
Enquiries -
lesleypocock@mediworld.com.au
.........................................................
While all
efforts have been made to ensure the accuracy
of the information in this journal, opinions
expressed are those of the authors and do not
necessarily reflect the views of The Publishers,
Editor or the Editorial Board. The publishers,
Editor and Editorial Board cannot be held responsible
for errors or any consequences arising from
the use of information contained in this journal;
or the views and opinions expressed. Publication
of any advertisements does not constitute any
endorsement by the Publishers and Editors of
the product advertised.
The contents
of this journal are copyright. Apart from any
fair dealing for purposes of private study,
research, criticism or review, as permitted
under the Australian Copyright Act, no part
of this program may be reproduced without the
permission of the publisher.
|
|
|
| August 2014 -
Volume 12 Issue 6 |
|
Seroprevalence
of HBV, HCV, HIV and syphilis infections among
blood donors at Blood Bank of King Hussein Medical
Center:
A 3 Year Study
Baheieh Al Abaddi
Maha Al Amr
Lamees Abasi
Abeer Saleem
Nisreen Abu hazeem
Ahmd Marafi
Princess Iman center for research and laboratory
sciences, King Hussein medical center, Jordan
Correspondence:
Dr. Lamees Abasi
Princess Iman center for research and laboratory
sciences, King Hussein medical center, Jordan
Email:
lameesabasi@yahoo.com
|
Abstract
Objective:
This retrospective study was performed
to find out the Seroprevalence of HBV,
HCV, HIV and syphilis infections among
blood donors at Blood Bank of King Hussein
medical center and to establish strict
guidelines for blood transfusion to reduce
the incidence of TTI, thus ensuring safe
blood supply to the recipients.
Method: The
present study was carried out in Blood
Bank of King Hussein Medical Center over
3 years from January 2009 through to December
2011. We determined among voluntary and
replacement blood donors at Princess Iman
Center for research and laboratory medicine,
the seroprevalence of human immunodeficiency
virus (HIV), hepatitis C virus (HCV),
hepatitis B virus (HBs Ag, HBc Ab) and
syphilis. Sera of all donors were tested
using commercial kits relying on enzyme
linked Immunosorbent assay. Qualitative
detection of HBs Ag was carried out using
(Bioelisa). Each donor's serum sample
was screened for HIV-1 and HIV-2 Ab using
Biorad (GenscreenHIV1/2 version2), and
HCV Ab screening is carried out using
Murex anti HCV version 4 following the
manufacturer's instructions. For in vitro
diagnostic use the IMMULITE 2000 systems
analyzers for the qualitative detection
of total antibodies against hepatitis
B core antigen (HBc Ab-total) in human
serum was used. Screening for Syphilis
was carried out using RPR (Rapid plasma
regains) confirmed by TPHA (Treponema
pallidum hemagglutination). Tests were
performed according to the manufacturer's
instructions.
Results: A
total of 94,270 blood donor records from
year 2009 to 2011 at King Hussein Medical
Center were apparently healthy adult voluntary
and replacement donors. Voluntary donors
represent 30% of the total donors while
replacement donors represent 70%. Total
number of 94,270 blood donors from year
2009 to 2011 at King Hussein Medical Center
were screened for HBs Ag, HCV Ab, HIV1/2
Ab, HBc Ab total and RPR at Princess Iman
Center for research and laboratory medicine.
In 2009 a total number of 28,315 were
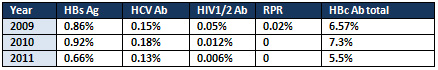
screened for TTI and show 245(0.86%) were
positive for HBs Ag, 44(0.15%) were positive
for HCV Ab, 13 (0.05%) were positive for
HIV1/2 Ab, 6(0.02%) were positive for
RPR, 1861 (6.57%) were positive for HBc
Ab total.
In 2010 a total number of 31,543 were
screened for TTI and show 293(0.92%) were
positive for HBs Ag, 57(0.18%) were positive
for HCV Ab, 4 (0.012%) were positive for
HIV1/2 Ab, zero % were positive for RPR,
and 2,305 (7.3%) were positive for HBc
Ab total.
In 2011 a total number of 34,412 were
screened for TTI and show 227(0.66%) were
positive for HBs Ag, 44(0.13%) were positive
for HCV Ab, 2 (0.006%) were positive for
HIV1/2 Ab, zero % were positive for RPR,
and 1,908 (5.5%) were positive for HBc
Ab total.
The overall prevalence of HBs Ag, HCVAb,
HIV1/2 Ab, RPR and HBc Ab total were 0.8%,
0.15%, 0.02%, 0.006%, and 6.4% respectively.
Conclusion:
This study shows that a substantial
percentage of the blood donors harbor
HIV, HBV, HCV and syphilis infections
so the use of sensitive screening test
for these TTI and establishment of strict
guidelines for blood transfusion are highly
recommended to reduce the incidence of
them, thus ensuring safe blood supply
to the recipients.
Key words:
Transfusion transmitted infection, Human
immunodeficiency virus, Hepatitis B virus,
Hepatitis C virus, Syphilis
|
Blood transfusion services (BTS) is an essential
part of the health care system; it ensures adequacy,
efficiency and safety of blood supply. (1)
Blood transfusion is a life saving procedure which
carries a major risk. Transfusion of Blood is
associated with many complications, some of which
are minor while others are life threatening that
need more proper pretransfusion testing and screening.
To improve blood transfusion safety, The World
Health Organization (WHO) recommends an incorporated
protocol that includes establishing a well-organized
blood transfusion service, giving the priority
of blood donation from voluntary unpaid donors,
screening of donated blood for the major transfusion-transmissible
infections (TTI) with quality-assured assays,
and applying effective quality control systems.
(2)
There is a 1% chance of transfusion associated
complications including transfusion transmitted
infections (TTI) with every unit of blood transfused.
(3)
The major concern of blood transfusion transmitted
infections is Human immunodeficiency virus (HIV),
hepatitis B virus (HBV) and hepatitis C virus
(HCV) because they can cause life-threatening
disorders. (4)
Syphilis is also a systemic disease caused by
Treponema pallidum. Transfusion transmitted infections
are a major concern to patients and physicians
who wish for a safe blood supply. Proper selection
of blood donors with low TTI risk and efficient
laboratory screening play a critical role in reducing
the risk of TTI in the last 20 years. (5, 6)
The aim of the present study was to find out prevalence
of transfusion transmitted infections (TTI) in
voluntary and replacement donors in our hospital
transfusion service set up. This study also aids
in evaluating the safety of the collected donations.
The present study was carried out in Blood Bank
of King Hussein Medical Center over 3 years from
January 2009 through to December 2011. We determined
among blood donors at Princess Iman Center for
research and laboratory medicine the seroprevalence
of human immunodeficiency virus (HIV), hepatitis
C virus (HCV), hepatitis B virus and syphilis.
Sera of all donors were tested using commercial
kits relying on enzyme linked Immunosorbent assay
for HBV, HCV and HIV, and using RPR for syphilis.
Qualitative detection of HBs Ag was carried out
using (Bioelisa). Each donor's serum sample was
screened for HIV-1 and HIV-2 Ab using Biorad (Genscreen
HIV1/2 version2), and HCV Ab screening is carried
out using Murex anti HCV version 4 following the
manufacturer's instructions.
For in vitro diagnostic use the IMMULITE 2000
systems analyzers for the qualitative detection
of total antibodies against hepatitis B core antigen
(HBc Ab-total) in human serum was used. IMMULITE
Anti-HBc Ag controls are assayed, tri-level controls
intended for use with the immulite 2000 Anti-HBc
Ag assays. Negative control: containing human
serum non reactive to HBc Ag, low positive control
and positive control containing human serum reactive
to HBc Ag.
Screening for Syphilis is carried out using RPR
(Rapid plasma regains) confirmed by TPHA (Treponema
pallidum hemagglutination). Tests were performed
according to the manufacturer's instructions.
All the reactive samples were repeated in duplicate
before labeling them seropositive. The donated
blood was discarded whenever the donor sample
was found positive for any TTI.
A total of 94,270 blood donor records from year
2009 to 2011 at King Hussein Medical Center were
apparently healthy adults 30% voluntary (motivated
blood donor, who donates at regular intervals)
and 70% replacement (usually one time blood donor
only when a relative is in need of blood). Blood
donors who were included in the study were healthy
men and non-pregnant non lactating women between
18 and 69 years, with hemoglobin levels above
13.5 g/d1 for males and 12.5 g/d1 for females
and weighing > 50 kg. Exclusion criteria
included: those with a history of jaundice, serious
illness, operation, radiotherapy or any form of
cancer therapy, current history of medication,
blood transfusion.
The largest proportion of donors (39%) were
in the age ranging from 35-50 years as shown
in Table 1. 87% of donors were males while 13%
were females.
Table 1: Age distribution for blood donors

As shown in Table 2 and Table 3, a total
number of 94,270 blood donors from year 2009
to 2011 at King Hussein Medical Center were
screened for HBs Ag, HCV Ab, HIV1/2 Ab, RPR
and HBc Ab total at Princess Iman Center for
research and laboratory medicine.
In 2009 a total number of 28,315 were screened
for TTI and show 245(0.86%) were positive for
HBs Ag, 44(0.15%) were positive for HCV Ab,
13 (0.05%) were positive for HIV1/2 Ab, 6(0.02%)
were positive for RPR, and 1,861 (6.57%) were
positive for HBc Ab total.
In 2010 a total number of 31,543 were screened
for TTI and show 293(0.92%) were positive for
HBs Ag, 57(0.18%) were positive for HCV Ab,
4 (0.012%) were positive for HIV1/2 Ab, zero
% positive for RPR, and 2,305 (7.3%) were positive
for HBc Ab total.
In 2011 a total number of 34,412 were screened
for TTI and show 227(0.66%) were positive for
HBs Ag, 44(0.13%) were positive for HCV Ab,
2 (0.006%) were positive for HIV1/2 Ab, zero
% positive for RPR, and 1,908 (5.5%) were positive
for HBc Ab total.
Table 2: TTI among blood donors (2009-2011)
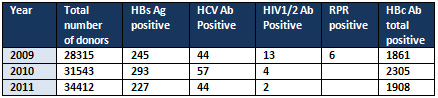
Table 3: Prevalence of TTI among blood donors
(2009-2011)
As shown in Table 4 the overall prevalence of
HBs Ag, HCVAb, HIV1/2 Ab, RPR and HBc Ab total
were 0.8%, 0.15%, 0.02%, 0.006%, and 6.4% respectively.
Table 4: Overall prevalence of TTI over
3 years' study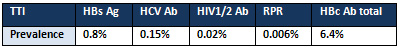
All the blood units with positive results for
HBs Ag, HCVAb, HIV1/2 Ab, RPR and HBc Ab total
were discarded.
Hepatitis B surface Antigen (HBs Ag) is the most
common method used to detect hepatitis B infection,
but using this marker alone in diagnosis of hepatitis
B infection is not efficient because it is not
detected during the window phase, so other markers
of HBV infection are used. This will prevent the
risk of transmitting hepatitis B infection.
Anti-HB core total is the marker that is used
as a screening test for hepatitis B virus infection
in the window phase. This test detects the presence
of both IgM and IgG antibody to hepatitis B core
antigen. This marker appears at the onset of symptoms
and persists for life.
As the results in Tables 1 and 2 show that the
prevalence of HB c Ab total positive results is
much higher than that of HBs Ag positive results,
so the number of blood units that were deferred
depending on this result is high.
Donated blood should be screened for HCV using
HCV Ab that can detect more than 95% of chronic
infection but can detect only 50-70% of acute
infection. As shown in Table 4 the overall prevalence
of HCV Ab was 0.15%.
The Acquired Immunodeficiency Syndrome (AIDS)
is caused by human immunodeficiency viruses, HIV-1
and HIV-2. Infection by HIV-1 has a worldwide
distribution while HIV-2 infection occurs mainly
in West Africa and Europe (7). It is necessary
for screening purposes to use antigens from the
envelope glycoproteins of both viruses, because
they are less cross reactive in addition to the
major cross reactive core proteins, to ensure
detection of antibodies against both types of
virus at all stages following infection (8).
The first specific antibody response for HIV infection
may be of immunoglobulin M (IgM) then immunoglobulin
G (IgG) (9). Maximum sensitivity for detection
of anti-HIV seroconversion is achieved by assays
to both IgM and IgG.
RPR is a rapid screening test for syphilis; all
positive results should be confirmed and the reactive
blood units should be deferred.
The blood units found positive for HBs Ag, HBc
AB, HCV Ab, HIV1/2 Ab and syphilis were discarded
and those donors were contacted via their phone
numbers included in the health questionnaire;
this is the approved policy for donor notification
in our center.
This study shows that a substantial percentage
of the blood donors harbor HIV, HBV, HCV and syphilis
infections so the use of sensitive screening test
for these TTI and establishment of strict guidelines
for blood transfusion are highly recommended to
reduce the incidence of TTI, thus ensuring safe
blood supply to the recipients.
1- World Health Organization:
Universal access to safe
blood transfusion. World
Health Organization, Geneva
; 2008.
2-World Health Organization:
Aide-mémoire: Blood
safety.World Health Organization,
Geneva ; 2002
3-Arora D, Arora B, Khetarpal
A (2010) Seroprevalence
of HIV, HBV, HCV and syphilis
in blood donors in Southern
Haryana . Indian J Pathol
Microbiol 53:308-309
4-UNAIDS: Report on the
global AIDS epidemic.
Geneva , Joint United
Nations program on HIV/AIDS;
2002.
5- Dodd RY: Current risk
for transfusion transmitted
infections. Curr Opin
Hematol 2007, 14:671-676.
6-Maresch C, Schluter
PJ, Wilson AD, Sleigh
A: Residual infectious
disease risk in screened
blood transfusion from
a high-prevalence population:
Santa Catarina, Brazil
.Transfusion 2008, 48:273-281.
7-. Clavel, F. (1987).
HIV-2: the West African
Aids virus. AIDS. 1, 135.
8-. Denis, F., Leonard,
G., et al, (1988). Comparison
of 10 enzyme immunoassays
for the detection of antibody
to human immunodeficiency
virus type 2 in West African
sera. J. Clin. Microbiol.
26, 1000.
9- Gains, H., von Sydons,
H., et al., (1988). Detection
of immunoglobulin M antibody
in primary human immunodeficiency
virus infection. AIDS,
2, 11.
|
|
.................................................................................................................

|
| |
|

