|
Seroprevalence of Measles,
Rubella, Mumps and Varicella Specific
Antibodies in Primary School Children
Reda Sanad Arafa
(1)
Ghada Saad Abdelmotaleb (1)
Raneyah Hamdy Mahmoud Shaker (2)
Inas Abdulmonem Elsayed (3)
Rabab Fawzy Salim Baioumy (3)
Nesreen Mohamad Zain El Dean (1)
Lamyaa Hussain Abdulrahman Seliem (1)
(1) Paediatric department, Benha faculty of
medicine, Benha University
(2) Public health department, Benha faculty
of medicine, Benha University
(3) Medical Biochemistry and Molecular Biology,
Benha Faculty of medicine, Benha University
Correspondence:
Dr Raneyah Hamdy Mahmoud Shaker
Assistant professor of public health
Public health and community medicine department
Benha faculty of medicine, Benha university,
Egypt
Email: prof_elashhab2003@yahoo.com,
rania.shaker@fmed.bu.edu.eg
|
Abstract
Measles,
mumps, rubella and varicella are diseases
that are tracked by the World Health Organization
(WHO) as common and serious vaccine-preventable
diseases.
Aim
of the Work: To evaluate the immune
status and susceptibility against measles,
mumps, rubella, and varicella in primary
school children and to study the effects
of some sociodemographic factors on the
seroprevalence.
Subjects
and methods: This is a cross- sectional
study conducted on 180 children. All children
included in this study were subjected
to thorough history taking and laboratory
investigations; to measure serum levels
of specific measles, rubella, mumps and
varicella immunoglobulins (IgG).
Results:
(88.9%) of the surveyed children were
seropositive to measles, (77.8%) to mumps,
(86.7%) to rubella and (38.9%) to varicella.
Seropositivity was higher in males than
in females for measles (57.7%), mumps
(60.7%), rubella (62.2%) and varicella
(68.6%) with significant difference for
measles. Younger age groups were less
seropositive than older age groups for
measles (32.5% vs 35%), mumps (34.4 %
vs 37.9%) , rubella ( 30.8 % vs 39.7 %)
and for varicella (21.4%) vs 48.6%). The
highest level of seronegativity was seen
with regard to varicella specific antibodies
(61.1%).
Conclusion and recommendations:
There is an urgent need for a planned
program with different strategies to prevent
and control these diseases .
Key words:
Seroprevalence, measles, mumps, rubella,
varicella, primary school children, Egypt
|
Measles is a highly contagious viral disease.
Typical symptoms are high fever, cough, coryza,
conjunctivitis and maculopapular rash. Common
complications include otitis media, Post-infectious
encephalitis in about 0.1% of reported cases,
and subacute sclerosing panencephalitis in about
1/10,000–100,000 cases.
Rubella is a viral disease presenting with fever,
rash and lymphadenopathy. Its importance is
caused by its teratogenic effect on the fetus
causing miscarriage, fetal death and congenital
rubella syndrome(2).
Mumps is a vaccine preventable viral infection.
Its typical clinical manifestations are pain
and swelling of the salivary glands, fever,
and fatigue. Other organs are commonly affected
(orchitis, oophoritis, pancreatitis, meningitis)
(3).
Varicella-zoster virus (VZV) is the etiologic
agent of varicella (primary infection) and herpes
zoster (reactivation of latent infection). Although
varicella is most often a relatively benign
and self-limited childhood illness, the disease
may be associated with a variety of serious
and potentially lethal complications in both
immunocompetent and immunocompromised persons
(4).
The measles, mumps and rubella ( MMR) vaccine
is a mixture of measles, mumps and rubella live
attenuated viruses, administered via SC injection.
The shot is generally administered to children
around the age of one year. The WHO recommends
that in order to eliminate congenital rubella
syndrome and to prevent the complications associated
with mumps and measles, countries should use
the measles, mumps and rubella (MMR) vaccine
in a two-dose schedule for routine childhood
immunization programs (5).
In 2002, Egypt established a goal of measles
elimination by 2010 using the WHO/UNICEF Comprehensive
Strategy for Sustainable Measles Mortality Reduction
(6) and also set a goal of rubella elimination
and congenital rubella syndrome (CRS) prevention
by 2010. The strategy for rubella elimination
included the introduction of MMR as the second
dose of measles-containing vaccine(MCV) in 1999.
In 2008, the immunization schedule was updated
to use MMR for both doses of MCV and to administer
the first dose at 12 months of age and the second
dose at 18 months of age (7).
A two dose program with varicella vaccination
is also likely to be required for elimination
of childhood varicella and has been recently
recommended in the United States(8).
The measles, mumps, rubella, and varicella (MMRV)
vaccine was licensed in 2005 for use among children
aged 12 months up to 12 years. It is a single
shot that can be used in place of two other
vaccines administered in two separate shots-the
measles, mumps, rubella (MMR) vaccine and the
varicella vaccine for chickenpox(9).
In Egypt, there is limited data about the serological
status of school children for these infectious
diseases. Monitoring school childrens' seroprevalence
and understanding the immune status of children
remains important to potentially identify those
with higher susceptibility and guide national
immunization policies to modify a routinely
administered schedule or implement a new schedule.
To evaluate the immune status and susceptibility
of a sample of primary school children against
measles, mumps, rubella, and varicella in Egypt
by conducting a seroprevalence survey utilizing
an enzyme immunoassay and to study the effects
of some sociodemographic factors on the seroprevalence.
This
is
a
cross-
sectional
study
conducted
at
Meat-Mousa
village
school,
Menoufia
Governorate,
Egypt
in
the
period
from
June
2013
up
to
February
2014.
Cluster
sampling
technique
was
used.
One
class
was
randomly
selected
from
each
educational
grade.
It
was
conducted
on
180
children;
their
ages
ranged
from
6
to
12
years.
They
were
109
males
and
71
females.
Parents
of
participants
were
asked
to
fill
out
an
especially
designed
questionnaire.
The
studied
children
were
subdivided
into
the
following
groups
according
to
their
ages:
-
Group
I
6
-8
years
(n=60)
-
Group
II
>8-10years
(n=56)
-
Group
III
>10-12
years
(n=64)
Inclusion
Criteria:
Apparently
healthy
Children
aged
6-12
years.
Exclusion
Criteria:
Acute
illness;
fever
more
than
38
degrees
centigrade
,recent
administration
of
immunoglobulins,
blood
product
or
immunosuppressive
therapy
and
suspected
or
confirmed
immune
suppressive
conditions.
The
study
was
conducted
according
to
the
rules
of
Benha
Faculty
of
Medicine
ethical
committee.
A
written
consent
from
all
student
parents
was
taken
with
explanation
of
the
purpose
of
the
study
and
ensuring
privacy.
All
children
included
in
this
study
were
subjected
to
the
following:
1-Thorough
history
taking:
•
Full
medical
history
including:
-
Full
personal
and
social
history
e.g.:
age,
sex,
residence,
order
of
birth.
-
Nutritional
history:
feeding,
breast
fed
or
bottle
fed.
-
Developmental
history:
motor
and
mental
development.
-
Vaccination
history
especially
MMR
timing
and
number
of
doses.
-
Contact
to
measles,
rubella,
mumps
and
varicella
cases
or
catch
up
the
diseases.
•
Parents
history
including:
-
Mother
and
father's
occupation
and
educational
degree
.
-
Socioeconomic
status
according
to
the
following
score(10)
:
The
total
score
was
| Scores
from
19-25
|
High
social
standard |
| Scores
from
12-18 |
Moderate
social
standard |
| Scores
from
6-11 |
Low
social
standard |
| Scores
of
<
6
|
Very
low
social
standard |
2-
Thorough
clinical
examination:
-
Anthropometric
measures
include:
weight,
height
and
body
mass
index.
-
Chest,
cardiac
and
abdominal
examination.
3-Laboratory
investigations:
About
3ml
of
peripheral
blood
was
withdrawn
from
each
child
into
a
sterile
vacutainer
and
allowed
to
clot.
After
centrifugation
the
obtained
sera
were
aliquoted
and
kept
frozen
at
-
20oC
till
further
processing.
The
serum
samples
were
used,
according
to
the
instructions
of
the
manufacturers
for:
1-
Measurement
of
serum
levels
of
specific
Measles
IgGusingKAPRMVG10
Measles
IgG
ELISA
kit
-DIAsource,
Belgium
(11).
2-
Mumps,
and
varicella
specific
IgG
were
done
for
all
children
using
ELISA
kits
(The
KAPRMUG12
Mumps
IgG
ELISA
kit
-DIA
source-Belgium
and
KAPRVIG20
Varicella
zoster
IgG
ELISA
kit
-
DIA
source-Belgium
(12).
3-
Measurement
of
serum
levels
of
specific
Rubella
IgG
using
RB025G
Rubella
IgG
ELISA
kit
-Calbiotech-
Spring
Vally)
(13).
The
Cut-Off
was
calculated
.The
sample
was
considered:
| Positive: |
If
the
ratio
was
>
1.1.
|
| Doubtful: |
If
+/-
10%
of
the
Cut-Off. |
| Negative: |
If
the
ratio
was
<
0.9. |
If
the
result
was
doubtful,
the
test
was
repeated.
If
it
was
still
doubtful,
a
new
serum
sample
was
collected.
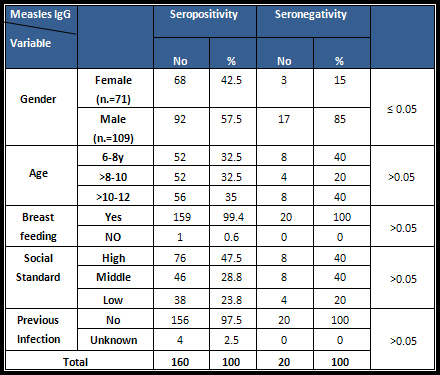
Table
1:
Seroprevalence
status
of
measles
antibodies
by
sociodemographic
factors
Table
2:
Seroprevalence
status
of
mumps
antibodies
by
sociodemographic
factors

Statistical
analysis
:
the
collected
data
were
tabulated
and
analyzed
using
(SPSS
version
16)
software
(SPSS
Inc.,
Chicago,
ILL
Company)
.
Chi-square
and
Fisher's
exact
are
statistical
tests
used
in
analysis
The
accepted
level
of
significance
will
be
(P
<
0.05
)
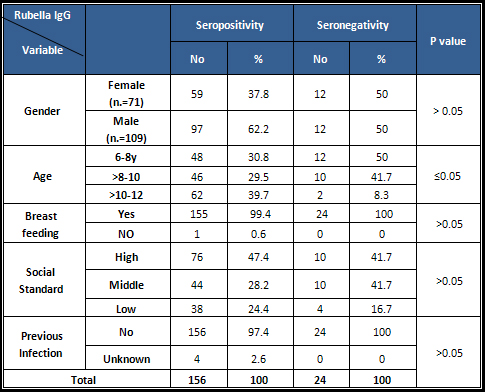
Table
3:
Seroprevalence
status
of
rubella
antibodies
by
sociodemographic
factors
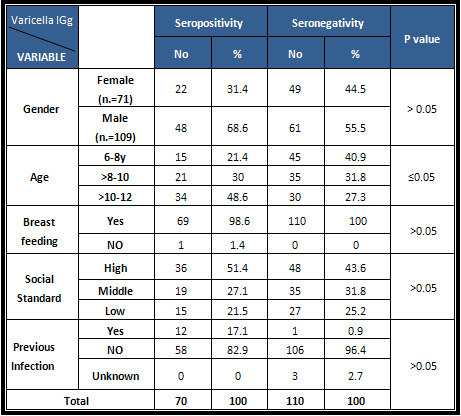
Table
4:
Seroprevalence
status
of
varicella
antibodies
by
sociodemographic
factors
A
total
of
180
children
were
surveyed
.
There
were
(60.6%)
males
and
(39.4%)
females.
The
age
ranged
from
6
up
to
12years
and
distributed
as
33.3%
for
6-8
age
group,
31.1%
for
>
8
-10
age
group
and
35.6%
%
for
>10-
12
age
group.
Only
18.3%
of
the
studied
children
of
age
group
(6-8
yrs)
received
the
MMR
vaccine
first
dose
(at
12
months)
while
all
of
the
other
2
groups
(>8-10
yrs
and
>10-12
yrs)
didn't
receive
the
first
dose
of
the
vaccine
at
12
month.
Regarding
the
second
dose
of
the
vaccine
(at
18
months)
all
age
groups
received
the
vaccine.
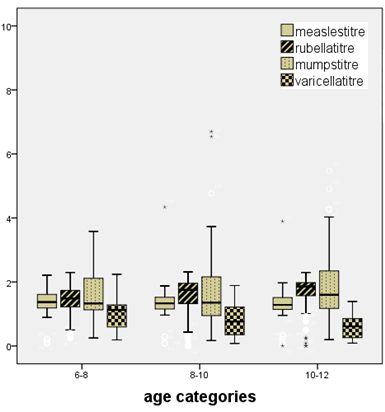
There
is
increasing
titre
of
measles,
mumps
and
rubella
IgG
with
increasing
age
with
no
statistical
significant
difference
but
there
is
a
statistically
significant
decreasing
varicella
titre
with
increasing
age.
Figure
(1)
Figure
1:
Distribution
of
titres
between
different
age
groups
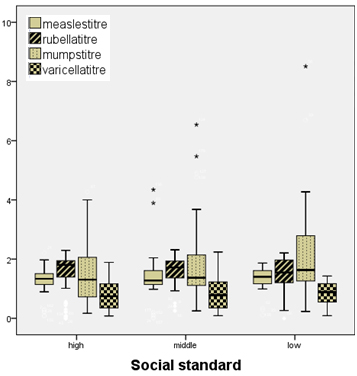
There
is
increasing
titre
of
measles
,mumps
and
varicella
among
low
social
class
while
rubella
titre
reported
higher
figures
among
high
social
class.
Figure
(2)
Figure
2:
Distribution
of
titres
between
different
social
classes
Regarding
seroprevalence
of
virus
antibodies
of
the
children
tested
160
(88.9%)
were
seropositive
to
measles,
140
(77.8%)
to
mumps,
156
(86.7%)
to
rubella
and
70
(38.9%)
to
varicella.
Seropositivity
was
higher
in
male
than
in
female
children
for
measles
(57.7%),
mumps
(60.7%),
rubella
(62.2%)
and
varicella
(68.6%).
No
significant
difference
was
found
between
male
and
female
with
regard
to
seropositivity
to,
mumps,
rubella
and
varicella;
however,
males
were
significantly
more
seropositive
to
measles
than
females
Analysis
of
the
data
according
to
age
reveals
that
there
were
significant
differences
in
seropositivity
rates
in
age
groups
for
mumps
,
rubella
and
varicella.
Younger
age
groups
were
less
seropositive
than
older
age
groups
for
measles
(32.5%
vs
35%),
mumps
(34.4%
vs
37.9%),
rubella
(
30.8%
vs
39.7%)
and
for
varicella
(21.4%
vs
48.6%)
with
statistically
significant
differences
except
for
measles.
The
majority
of
the
surveyed
children
were
normally
breast
fed
(99.4%)
with
no
significant
higher
seropositivity
rates
among
them
for
measles
(
99.4
%),
mumps
(99.3%),
rubella
(99.4%)
and
varicella
(98.6%).
Overall,
the
highest
level
of
seronegativity
was
seen
with
regard
to
varicella
specific
antibodies
(61.1%).
Some
differences
for
gender
and
age
were
seen;
In
general,
girls
had
a
lower
rate
of
seronegativity
for
measles
(15%),
mumps
(40%)
and
varicella
(
49%)
but
this
pattern
was
not
seen
in
rubella
(50%).
Generally,
seronegativity
was
highest
in
the
age
group
6-
8
year
old
children
for
measles
(
40%),
rubella
(50%),
and
varicella
(45%)
but
for
mumps
it
is
highest
among
8-10
age
group
(42.5%).
Higher
seropositivity
of
measles
(47.5%),
mumps
(50%),
rubella
(47.4%)
and
varicella
(51.5%)
IgG
were
observed
in
high
social
class
compared
to
middle
and
low
social
classes
but
with
no
statistical
significant
differences.
High
percentage
(97.5%,
87.9%,
97.4%
and
82.9%)
of
seropositivity
of
the
studied
group
was
in
children
without
past
history
of
infection
regarding
measles,
mumps,
rubella
and
varicella
IgG
respectively
with
no
significant
statistical
results.
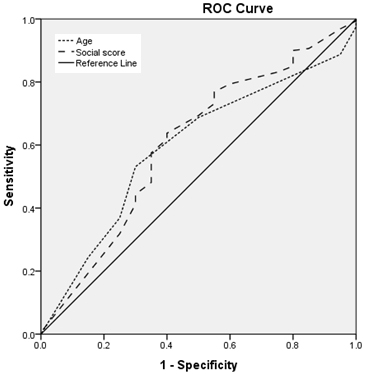
Figure
3:
Relation
between
measles
IgG
titre
and
age
and
social
class
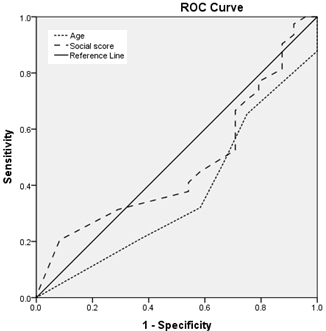
Figure
4
:
Relation
between
rubella
IgG
titre
and
age
and
social
class
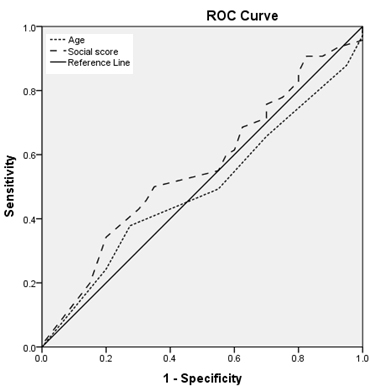
Figure
5:
Relation
between
mumps
IgG
titre
and
age
and
social
class
Figure
6:
Relation
between
varicella
IgG
titre
and
age
and
social
class
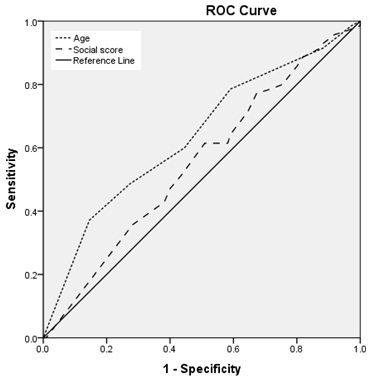
Figures
(3,4,5,6,):
Regarding
age:
Area
under
the
curve
is
:
less
than
0.6
for
rubella
and
mumps
(i.e.,
age
is
a
worthless
predictor
for
sero-
prevalence
of
rubella
and
mumps),
nearly
0.6
for
measles,
larger
than
0.6
for
varicella
(ie.,
age
is
a
fair
predictor
for
seroprevalence
of
measles
and
varicella)
with
statistically
significant
results
for
rubella
and
varicella.
Figures
(3,4,5,6)
Regarding
social
class
:
Area
under
the
curve
is:
equal
to
0.6
for
measles
(i.e.,
social
score
is
a
fair
predictor
for
sero-
prevalence
of
measles),
less
than
0.6
for
rubella,
mumps
and
varicella
(ie.,
Social
score
is
a
worthless
predictor
for
seroprevalence
of
rubella
mumps
and
varicella)
with
non
statistically
significant
results.
Measles,
mumps,
rubella
and
varicella
are
diseases
that
are
tracked
by
the
World
Health
Organization
(WHO)
as
common
and
serious
vaccine-preventable
diseases
i.e
;
licensed
vaccines
are
available
to
prevent,
or
contribute
to
the
prevention
and
control
of
them.
Immunization
is
one
of
the
safest,
most
cost-effective
means
of
preventing
diseases.
Nowadays,
all
countries
of
the
world
have
incorporated
a
broad
immunization
program
in
their
public
health
interventions.
Our
study
showed
that
88.8
%
of
the
studied
group
was
seropositive
for
measles
IgG
and
86.6%
was
seropositive
for
rubella
IgG.
These
results
were
in
agreement
with
different
studies;
in
Egypt
and
Pakistan,
86.1%
and
79.9%
respectively
of
the
studied
group
was
seropositive
for
measles
IgG
(14,15).
In
Germany
seropositivity
was
89.5%
for
measles
and
86.25%
for
rubella
(16)
In
Thailand
and
Iran
93.4%
and
85%
respectively
were
seropositive
for
rubella
(17,
18).
This
study
revealed
that
77.8%.
were
seropositive
for
mumps
IgG.
Different
figures
have
been
reported
world
wide;
in
the
United
States
88%
positivity
for
two
doses(19,20)
and
overall
94%
positivity
were
reported
in
another
study
(21)
and
in
Bulgaria
79%
were
positive(3).
This
study
reported
that
the
percentage
of
seropositivity
of
varicella
IgG
was
only
38.9%.
In
contrary
to
our
results
a
higher
rate
was
reported
in
other
studies;
in
Bangladesh
seropositivity
is
approximately
65%
(22),
In
Australia;
83%
by
the
ages
of
10-14
years
(23)
and
in
Turkey
and
Italy,
levels
of
immunity
by
the
ages
of
10-14
years
were
85%
and
82
%
respectively(24,
25).
Differences
between
countries
are
likely
to
be
related
to
climatic
conditions
and
mixing
patterns,
particularly
in
relation
to
child
day
care
(23)
or
difference
in
vaccination
program
coverage.
There
are
some
differences
between
regional
seropositivity
rates
which
are
perhaps
attributable
to
differences
in
the
design
of
early
childhood
immunization
programmes
of
each
country.
This
study
shows
that
seropositivity
was
higher
in
male
than
in
female
children
for
measles
(57.7%),
mumps
(60.7%),
rubella
(62.2%)
and
varicella
(68.6%)
with
no
significant
results
except
for
measles.
For
measles
this
was
in
agreement
with
many
studies
in
Pakistan
(15)
and
in
Korea
(26)
but
it
was
in
contrast
with
others
(14,
16,
27).
For
rubella
this
was
in
contrast
with
others
in
Germany
(16)
in
Japan
(27)
and
in
Colombia
(28).
Regarding
mumps,
our
results
coincide
with
a
German
study
which
revealed
that
boys
were
more
likely
to
be
seronegative
to
measles,
mumps
and
rubella
than
girls
(16).
On
the
other
hand
a
study
conducted
on
Bulgarian
children
revealed
a
significantly
higher
prevalence
of
mumps
antibodies
in
girls(29).
Measles
was
endemic
in
Egypt
until
2008.
During
the
1980s,
large
measles
epidemics
occurred
every
2-4
years.
Similarly,
outbreaks
in
the
1990s
continued
to
occur
every
2-4
years.
Between
1996
and
2000,
the
majority
(>80%)
of
measles
cases
were
reported
in
persons
aged
>10
years
(30).
Since
2000,
there
has
been
a
remarkable
decrease
in
the
number
of
reported
cases
of
measles.
This
decrease
has
occurred
among
age-groups
targeted
by
the
mass
vaccination
campaigns
conducted
during
2000-2004
as
a
part
of
the
measles
elimination
strategy.
In
2006,
however,
the
number
of
confirmed
measles
cases
increased
dramatically
to
953
.
In
2006,
the
age
distribution
of
cases
was
as
follows:
22%
aged
1-5
years,
56%
aged
6-15
years,
and
10%
aged
16-20
years
(7).
In
2008,
reported
measles
cases
decreased
to
771
with
a
similar
age
distribution
.
In
2008
and
2009
a
2-phase
measles,
rubella
(MR)
campaign
was
conducted
and
had
a
significant
impact
on
measles
cases(2).
Rubella
surveillance
was
part
of
communicable
diseases
surveillance
in
Egypt
and
had
been
in
place
for
many
years
(7).
In
2002
and
2003,
274
and
261
confirmed
rubella
cases
were
reported,
respectively,
of
which
many
(>45%)
occurred
among
children
5-9
years
of
age.
In
2005
up
to
2007,
a
nationwide
epidemic
began;
most
rubella
cases
were
reported
among
persons
11-20
years
old.
In
2008,
the
epidemic
waned
.
Following
the
2008-2009
MR
vaccination
campaign,
only
a
few
cases
of
rubella
were
reported
(2).
Also
in
a
recent
study
in
Egypt
the
overall
measles
antibody
seropositivity
was
88%
and
rubella
antibody
seropositivity
was
74%.
Measles
antibody
seropositivity
averaged
87%
in
1-
to
4-year
old
children
and
increased
to
an
average
of
93%
in
children
aged
10-20
years.
Rubella
antibody
seropositivity
averaged
56%
(range:
43-71%)
in
children
aged
1-4
years
and
gradually
increased
to
an
average
of
91%
in
adolescents
and
young
adults
aged
15-19
years
old
(6).
This
coincides
with
the
results
of
this
study
which
reveals
that
younger
age
groups
were
less
seropositive
than
older
age
groups
with
significant
differences
in
seropositivity
rates
in
age
groups
for
mumps
,
rubella
and
varicella.
The
increasing
prevalence
of
antibodies
in
the
older
children
may
be
due
to
either
vaccination
schedule
or
exposure
to
natural
infection/
mature
immune
systems.
Regarding
measles,
this
was
in
agreement
with
others
(14,
15)
but
in
contrast
with
another
study
in
Germany
(16)
which
reported
a
higher
percentage
in
7-10
age
groups
than
in
11-13
years
old
.
Also
the
increasing
measles
antibody
level
by
age
was
supported
by
many
researchers;
in
Korean
children
(26),
in
Italian
children
(31),
WHO
Report
(32)
and
in
Australian
children
(33,15).
Regarding
rubella,
this
was
in
agreement
with
Tharmaphornpilas
(2009)
study
in
Thailand
(17),
but
in
contrast
with
others
(16).
In
our
study,
age
of
children
had
a
significant
effect
on
the
seropositivity
of
mumps
titre.
Age
was
reported
as
a
significant
factor
by
many
studies.
In
a
Bangladeshi
population
mumps
antibody
had
shown
a
steep
rise
from
age
2
to
3
years
up
to
14-15
years
age
(34).
On
the
other
hand,
in
another
study
on
Finnish
children
there
were
declining
mumps
antibody
levels
and
rising
negativity
rates
(35).
In
this
study,
varicella
seronegativity
decreased
significantly
with
age
of
the
studied
children
group.
This
coincides
with
a
seroepidemiologic
survey
in
Catalonia
(Spain)
which
reported
decreased
susceptibility
to
VZV
by
increasing
age
(36)
but
disagrees
with
other
studies
in
Canada
(37)
and
in
Sri
Lanka
(38).
Also
a
study
conducted
in
Saudi
Arabia
revealed
a
non
significant
difference
between
age
groups
in
the
prevalence
of
immunity
to
varicella
(39).
Regarding
the
type
of
feeding,
our
study
showed
that
breast
feeding
has
no
significant
effect
on
measles
,
mumps
or
rubella
IgG.
The
same
results
were
obtained
by
others
(40,
41).
According
to
the
socioeconomic
state,
higher
seropositivity
of
measles
(47.5%),
mumps
(50%),
rubella
(47.4%)
and
varicella
(51.5%)
IgG
were
observed
among
high
social
class
compared
to
middle
and
low
social
classes
but
with
no
statistical
significant
differences.
This
was
in
agreement
with
Abu
Zaid
study,
(41)
which
reveals
non
significant
differences
between
social
levels.
Our
study
showed
that
most
of
the
studied
vaccinated
children
had
no
history
of
measles,
rubella
or
mumps
infections.
This
is
also
supported
by
the
Poethko
Muller
and
Mankertz
study
in
Germany
(16).
The
seroprevalence
survey
studies
had
important
implications
for
the
management
of
vaccine
programs
which
contributes
to
the
prevention
of
disease
transmission.
In
this
study
seropositivity
was
higher
in
male
than
in
female
children
with
a
significant
difference
for
measles.
Younger
age
groups
were
less
seropositive
than
older
age
groups
with
significant
differences
except
for
measles.
The
highest
level
of
seronegativity
was
seen
with
varicella
specific
antibodies.
Higher
seropositivity
was
observed
in
high
social
class
and
in
children
without
past
history
of
infection
with
no
significant
statistical
results.
Immunization
programs
face
many
challenges:
to
introduce
new
vaccines,
to
achieve
and
sustain
high
coverage
for
those
already
in
the
program.
There
is
a
need
for
a
planned
program
to
prevent
and
control
these
diseases
with
the
following
strategies:
•
Adding
a
3rd
dose
of
MMR
vaccine
at
age
4-6
years
old
to
increase
the
protective
efficiency
of
vaccine
and
to
be
sure
of
the
elimination
of
the
diseases
at
the
adolescence
period.
•
Introduction
of
MMRV
vaccine
instead
of
MMR
for
all
children
to
avoid
infection
and
serious
complications,
especially
in
older
ages
of
children
without
or
of
unknown
history
of
previous
infection.
•
Testing
older
children
for
varicella
(IgG)
to
determine
their
vulnerability
to
vaccine
before
vaccination.
•
Conduction
of
planned
health
awareness
activities
directed
towards
more
orientation
about
Immunization.
•
Adopt
scientific
advice
on
vaccines
that
will
support
policy
makers
in
their
decisions
regarding
the
national
vaccination
schedules.
1-
WHO.
World
Health
Organization.
Reported
measles
cases
and
incidence
rates
by
WHO
Member
States
2013,
2014;
.Geneva,
WHO.
2-
WHO:
World
Health
Organization.
Rubella
vaccines:
WHO
position
paper
.Weekly
epidemiological
record
2011;
No.
29,
86,
301-316.
3-Karcheva
M,
Atanasova
M,
MakaveevI
and
Daskalova
M.
Study
on
seroprevalence
of
mumps
-
specific
IgG
antibodies
in
a
healthy
population.
Journal
of
IMAB
-
Annual
Proceeding
(Scientific
Papers)
2010;
vol.
16,
23-26.
4-
Gnann
J.
Varicella-Zoster
Virus
Atypical
Presentations
and
Unusual
Complications,
The
Journal
of
Infectious
Diseases.
2002;
Volume
186,
Issue
Supplement,
1:91-98.
5-Okonko
O,
Onoja
BA,
Adedeji
AO,
Ogun
A
A
et
al.,
The
role
of
vaccines
in
elimination
and
global
eradication
of
measles:
review
of
literature.
African
Journal
of
Pharmacy
and
Pharmacology
2009;
Vol.
3(9):
413-425.
6-
El
Sayed
N,
N.
Kandeel,
I.
Barakat,
et
al.,
Progress
Toward
Measles
and
Rubella
Elimination
in
Egypt
The
Journal
of
Infectious
Diseases
2011;
204:S318-S324.
7-
WHO.
World
Health
Organization.
Central
plan
of
action
for
Measles-Rubella
campaign
among
persons
aged
1-20
in
Egypt.
Phase
I.
Geneva
2008,
WHO.
8-Saad
A,
Safi-El-Dine
A
and
AI
El-Shamy
K.
The
Trend
of
Mandatory
Vaccination
among
Children
in
Egypt.
The
Open
Vaccine
Journal;
2009;2:77-84.
9-
Marin
M,
Broder
KR,
Temte
JL,
et
al.
Centers
for
Disease
Control
and
Prevention
(CDC).
Use
of
combination
measles,
mumps,
rubella,
and
varicella
vaccine:
Recommendations
of
the
Advisory
Committee
on
Immunization
Practices
(ACIP).
MMWR
Recomm
2010;
59(3):
1-12.
10-Fahmy
SI
and
El-Sherbini
AF.
Determining
simple
parameters
for
social
classifications
for
health
research.
Bull
High
Inst
Public
Health
1983;
235:1-14.
11-
Erdman
DD,
Heath
JL,
Watson
JC,
Markowitz
LE,
Bellini
WJ.
Immunoglobulin
M
antibody
following
primary
and
secondary
vaccination
and
natural
virus
infection.
J
Med
Virol
1993;41:44-8.
12-Berbers
GA,
Marzec
AH,
Bastmeijer
M,
van
Gageldonk
PG,
Plantinga
AD.
Blocking
ELISA
for
detection
of
mumps
virus
antibodies
in
human
sera.
J
Virol
Methods
1993;
42:
155-168.
13-
Matter
L;
Kogelschatz
K;
Germann
D.
Serum
levels
of
rubella
virus
antibodies
indicating
immunity:
response
to
vaccination
of
subjects
with
low
or
undetectable
antibody
concentrations.
J
Infect
Dis
1997;
175(4):749-55.
14-Tayil
S.E.,
Elshazly
M.K.,
El
Amrawy
S.M.
et
al.
Seroepidemiological
study
of
measles
after
15
years
of
compulsory
vaccination
in
Alexandria.
Eastern
Mediterranean
Health
Journal
1998;
vol.
4,
No.
3.P:
437-447.
15-
ChannaRA
,Hussain
S
,Kanher
NA
et
al.,
Sero-surveillance
of
measles
amongst
vaccinated
and
non-vaccinated
children:
An
age
stratified
population
based
survey
in
Pakistan.
African
Journal
of
Pharmacy
and
Pharmacology
2012;
Vol.
6(24)
:
1713-1718
.
16-
Poethko-Muller
C
and
Mankertz
A.:
Seroprevalence
of
Measles-,
Mumps-
and
Rubella-Specific
IgG
Antibodies
in
German
Children
and
Adolescents
and
Predictors
for
Seronegativity.
PLoS
ONE
2012;
7(8):
e42867.
17-
Tharmaphornpilas
P,
YoochareanP
,
-Rasdjarmrearnsook
AR
et
al.,:
Seroprevalence
of
Antibodies
to
Measles,
Mumps,
and
Rubella
among
Thai
Population:
Evaluation
of
Measles/MMR
Immunization
Programme.J
HEALTH
POPUL
NUTR
Feb
2009;
27(1):80-1606-0997
18-
Nanbakhsh
F,
S
Salari-Lak
,
F
Broomand
et
al.,:
Evaluation
of
Rubella
IgG
Antibodies
among
High
School
Girlsin
Uremia
City
Iranian
J
Publ
Health
2003;
Vol.
32,
No.
3,
pp33.-36.
19-
Schaffzin
JK,
Pollock
L,
Schulte
C,
Henry
K
et
al.,
Effectiveness
of
previous
mumps
vaccination
during
a
summer
camp
outbreak.
Pediatrics
2007;
120(4):e862-828.
20-
Harling
R,
White
JM,
Ramsy
ME
et
al.,
The
effectiveness
of
the
mumps
component
of
the
MMR
vaccine:
a
case
control
study.
Vaccine
2005;
23(31):4070-4074..
21-
Date
AA,
Kyaw
MH,
Rue
AM,
Klahn
J
et
al.,
Long-Term
Persistence
of
Mumps
Antibody
after
Receipt
of
2
Measles-Mumps-Rubella
(MMR)Vaccinations
and
Antibody
Response
after
a
Third
MMR
Vaccination
among
a
University
Population.
The
Journal
of
Infectious
Diseases
2008;197:1662-
1668.
22-Saha
SK,
Darmstadt
GL,
Hanif
M,
Khan
R
et
al.,
Sero
epidemiology
of
VZV
in
Bangladesh,
Annuals
of
Tropical
Pediatrics
2002;
22:341-345.
23-
Gidding
HF,
MacIntyre
CR,
Burgess
MA
and
Gilbert
GL.
The
seroepidemiology
and
transmission
dynamics
of
varicella
in
Australia.
Epidemiol
Infect.
2003;131:1085-1089.
24-Gabutti
G,
Penna
C,
Rossi
M,
et
al.,
The
sero
epidemiology
of
varicella
in
Italy.
Epidemiol
Infect
2001;
126:
433-440
.
25-
Kanra
G,
Isik
P,
Kanra
A,
et
al.,
Complementary
findings
in
clinical
and
epidemiologic
features
of
mumps
and
mumps
meningoencephalitis
in
children
without
mumps
vaccination.
Pediatr
Int
2004;
46:663-668
26-
Kim
SS,
Han
HW,
Go
U
et
al.,
Sero-epidemiology
of
measles
and
mumps
in
Korea:
impact
of
the
catch-up
campaign
on
measles
immunity.
Vaccine,
2004;
23:
290-297.
27-
Kumakura
S,
Shibata
H,
Onoda
K
et
al.,
Seroprevalence
survey
on
measles,
mumps,
rubella
and
varicella
antibodies
in
healthcare
workers
in
Japan:
sex,
age,
occupational-related
differences
and
vaccine
efficacy
.Epidemiol.
Infect.
(2014);
142(1)
12-19.
.
28-
Hincapie-Palacio
D,
Lenis
V,
Ballesteros
M
et
al.,
Seroprevalence
of
rubella
in
Colombia:
a
birth-year
cohort
analysis
Rev
SaúdePública
2013;47(6):1-1
29-
Karcheva
M
and
Gancheva
G.
Seroprevalence
of
IgG
antibodies
against
Mumps
in
Bulgarian
Children
under
18
Years
of
Age.
alkan
Med
J.
2013;
30(1):
16-18.
30-WHO.
World
Health
Organization.
Desk
review-Egypt-measles.
Geneva,
WHO.
2003.
31-Salmaso
S,
Gabutti
G,
Rota
MC
et
al.,
Pattern
of
susceptibility
to
measles
in
Italy.
Bulletin
of
the
2000;
78:
950-955.
32-
WHO.
World
Health
Organisation.
Vaccine,
Immunization,
and
Biological
Study:
Measles.
World
Health
Organization
publication.
November
.Geneva
.WHO.
2000.
33-
Gilbert
GL,
Escott
RG,
Gidding
HF
et
al.,
Impact
of
the
Australian
Measles
Control
Campaign
on
immunity
to
measles
and
rubella.
Epidemiol.
Infect.
2001;
127:
297-303.
34-
Sultana
R,
Rahman
MM,
Hassan
Z
and
Hassan
MS.
Prevalence
of
IgG
Antibody
Against
Measles,
Mumps
and
Rubella
in
Bangladeshi
Children:
A
Pilot
Study
to
evaluate
the
need
for
integrated
vaccination
strategy.
Scandinavian
Journal
of
Immunology
2006;
64:
6846-6868.
35-Davidkin
I,
Jokinen
I,
Broman
M,
Leinikki
P
et
al
.
Persistence
of
measles,
mumps,
and
rubella
antibodies
in
an
MMR-vaccinated
cohort:
a
20-year
follow-up.
J
Infect
Dis.
2008;
197:950-956.
36-Salleras
L,
Dominguez
A,
Vidal
J,
Plans
P,
Salleras
M,
Taberner
JL.
Seroepidemiology
of
varicella-zoster
virus
infections
in
Catalonia
(Spain):
rationale
for
universal
vaccination
programs.
Vaccine
2001;
19:
183-188.
37-Canadian
Pediatric
Society.
Preventing
varicella:
Recommendations
for
routine
two
dose
varicella
immunization
in
children
(Position
Statement).
Paediatr
Child
Health
2011;
16(7):
415.
38-
Bartoloni
A,
Bartalesi
F,
Roselli
M,
Mantella
A
et
al.,
Seroprevalence
of
varicella
zoster
and
rubella
antibodies
among
rural
populations
of
the
Chaco
region,
south-eastern
Bolivia.
Trop
Med
Int
Health
2002;
7:
512-517.
39-Almuneef
M.A,
Memish
Z.A,
Balkhy
H.H,
Otaibi
B
et
al.,
Seroprevalence
Survey
of
Varicella,
Measles,
Rubella,
and
Hepatitis
A
and
B
Viruses
in
a
Multinational
Healthcare
Workforce
in
Saudi
Arabia.
Infect
Control
Hosp
Epidemiol
2006;
27:1178-1183.
40-
Elers
H,
Abdel
Galil
TE,
and
Soliman
OE.
Seroprevalence
of
maternal
IgG
antibodies
against
measles,
mumps,
and
rubella
in
infants
in
Dakahlia
governorate.
Msc
Thesis
.Mansoura
university.
2005.
41-
Abu
Zaid
A
M
,
El-Shafie
N
.A.
,
Zaki
S.
M
et
al.,
Study
of
Age
Related
Significant
Decline
of
IgG
titer
For
Measles,
Mumps
and
Rubella
among
Infants
of
Sharkia
Governorate.MSC
Thesis.
Zagazig
university.
2006.
|

