|
|
 |
| ............................................................. |
|
|
| ........................................................ |
| From
the Editor |

|
Editorial
A. Abyad (Chief Editor) |
|
|
|
|
........................................................ |
Original
Contribution / Clinical Investigation
|



|
<-- Lebanon, Saudi Arabia, Algeria, UK,
Dubai, Qatar -->
The
dos and don’ts of painful diabetic peripheral
neuropathy: Primary care guidelines for the
Middle East and North Africa
[pdf
version]
Camille Aizarani , Ashraf A. Amir, Zoulikha
Benchouk, Muneer A. Abu Al-SamenMohamed Farghaly,
Adnan Kandil, Rayaz A. Malik
<-- Iran -->
Effect
of electro-acoustic factors on the continuous
use of hearing aid in hearing impaired children
under 15 years
[pdf
version]
Mansour Nazari Chafjiri, Nikta Hatamizadeh,
Asghar Makarem, Masoud Karimloo
<-- Iran -->
Comparative
study of the effects of narrative therapy and
play therapy by group approach on inhibiting
impulsivity, reducing aggression and increasing
interpersonal relations
[pdf
version]
Sepideh Kafili Kasmaei, Farhad Asghari
|
........................................................
International Health
Affairs
........................................................
|
Chief
Editor -
Abdulrazak
Abyad
MD, MPH, MBA, AGSF, AFCHSE
.........................................................
Editorial
Office -
Abyad Medical Center & Middle East Longevity
Institute
Azmi Street, Abdo Center,
PO BOX 618
Tripoli, Lebanon
Phone: (961) 6-443684
Fax: (961) 6-443685
Email:
aabyad@cyberia.net.lb
.........................................................
Publisher
-
Lesley
Pocock
medi+WORLD International
11 Colston Avenue,
Sherbrooke 3789
AUSTRALIA
Phone: +61 (3) 9005 9847
Fax: +61 (3) 9012 5857
Email:
lesleypocock@mediworld.com.au
.........................................................
Editorial
Enquiries -
abyad@cyberia.net.lb
.........................................................
Advertising
Enquiries -
lesleypocock@mediworld.com.au
.........................................................
While all
efforts have been made to ensure the accuracy
of the information in this journal, opinions
expressed are those of the authors and do not
necessarily reflect the views of The Publishers,
Editor or the Editorial Board. The publishers,
Editor and Editorial Board cannot be held responsible
for errors or any consequences arising from
the use of information contained in this journal;
or the views and opinions expressed. Publication
of any advertisements does not constitute any
endorsement by the Publishers and Editors of
the product advertised.
The contents
of this journal are copyright. Apart from any
fair dealing for purposes of private study,
research, criticism or review, as permitted
under the Australian Copyright Act, no part
of this program may be reproduced without the
permission of the publisher.
|
|
|
| June 2017 - Volume
15, Issue 4 |
|
|
The dos and don'ts of
painful diabetic peripheral neuropathy: Primary
care guidelines for the Middle East and North
Africa
Camille Aizarani
(1)
Ashraf A. Amir (2)
Zoulikha Benchouk (3)
Muneer A. Abu Al-Samen (4)
Mohamed Farghaly (5)
Adnan Kandil (6)
Rayaz A. Malik (7)
(1) Family Medicine
Specialist, Clemenceau Medical Center, Beirut,
Lebanon
(2) Consultant Family Medicine Specialist, International
Medical Center, Jeddah, Saudi Arabia
(3) General Physician, Ras El Ain Health Center,
Bouamama, Oran, Algeria
(4) Diabetologist, University of Leicester,
London, UK
(5) Family Medicine Specialist, Dubai Health
Authority, Dubai
(6) Head, Epidemiology and Preventive Medicine
Service, Zeralda Health Sector, Algiers, Algeria
(7) Professor of Medicine, Weill Cornell Medicine,
Qatar
Correspondence:
Dr Camille
Aizarani, MD
Clemenceau Medical Center,
Cairo Street,
Beirut,
Lebanon
Tel: 00961-3-810791
Email: draizarani@hotmail.com
|
Abstract
Background:
Diabetes mellitus (DM) is becoming
increasingly common in developing countries
and is of major concern in the Middle
East and North Africa (MENA). Since at
least 30% of diabetic patients may develop
painful diabetic peripheral neuropathy
(pDPN) within their lifetime, there is
an urgent need to increase awareness of
the condition among physicians in the
region.
Objectives:
To increase awareness of physicians in
the Middle East and North Africa of the
increasing prevalence of DM and pDPN and
to provide practical consensus recommendations
to facilitate the diagnosis and management
of pDPN.
Methods: A
panel of family medicine physicians was
convened in Dubai to discuss current awareness
of pDPN in the region and to develop consensus
statements based on a review of meta-analyses,
systematic reviews, and evidence-based
guidelines on the screening, diagnosis
and management of pDPN.
Recommendations:
The panel recommends that all patients
with diabetes be screened at least annually
for symptoms of neuropathic pain using
screening tools such as the Doleur Neuropathique
en 4 Questions (DN4) as well as thorough
examination of the patient's feet. Treatment
should aim to achieve a clinically meaningful
reduction in pain using first-line agents
including pregabalin, duloxetine or tricyclic
antidepressants.
Conclusion:
pDPN is common but under-diagnosed and
inadequately treated in the Middle East
and North Africa. Physicians in the region
are encouraged to implement screening
for pDPN and manage patients according
to published guidelines.
Key words:
Painful diabetic neuropathy, Middle East,
North Africa, neuropathic pain, consensus
recommendations
|
Once considered a 'disease of affluence', diabetes
mellitus (DM) is becoming increasingly common
in developing countries and is of major concern
in the Middle East and North Africa.(1) Complications
of diabetes include cardiovascular disease, nephropathy
and retinopathy, but the most commonly encountered
complication is diabetic peripheral neuropathy
(DPN),(2) and it is estimated that approximately
30% of diabetic patients have painful diabetic
peripheral neuropathy (pDPN).(2-6)
Clinically, DPN is a diagnosis of exclusion, defined
as "the presence of symptoms and/or signs
of peripheral nerve dysfunction in people with
diabetes after the exclusion of other causes".(7)
pDPN has a significant negative impact on quality
of life by reducing patients' mobility and ability
to perform everyday tasks, increasing the risk
of foot ulcers and amputation, disturbing sleep
and causing psychological distress.(3, 8)
pDPN is widely under-diagnosed and often poorly
treated. A survey conducted by the American Diabetes
Association in 2005 found that up to 75% of patients
who experienced pDPN symptoms were not diagnosed
and that 56% of these patients were not even aware
of the condition.(5) Other studies have also shown
that patients who do receive treatment are often
dissatisfied with the outcomes.(6, 9) The few
studies of pDPN that have been conducted in Middle
Eastern and North African countries suggest prevalence
ranges from 22-65%, reflecting different populations
and methods of diagnosing this condition, but
may also indicate true differences from the expected
prevalence of approximately 30%.(1, 10-12)
The present review and clinical guidelines aim
to increase awareness of pDPN among physicians
in the Middle East and North Africa as well as
to provide practical consensus recommendations
to facilitate the diagnosis and management of
pDPN in the region.
A panel of seven family medicine physicians
from the Middle East and North Africa, together
with Professor Rayaz A. Malik from Weill Cornell
Medicine in Doha, Qatar and NY, USA, was convened
in Dubai, United Arab Emirates, on 17 October
2015. The panel members had extensive clinical
and research expertise in the diagnosis and
management of pDPN in the general practice setting,
while Professor Malik, who is a member of the
writing group for the 2016 American Diabetes
Association consensus statement on diabetic
neuropathy, was an advisor to the panel. The
key objectives of the meeting were to gain a
better understanding of the most pressing challenges
facing family medicine practitioners in the
region regarding the management of pDPN in their
day-to-day clinical practice and to develop
consensus recommendations to optimize diagnosis
and treatment.
The panel discussed 6 pertinent themes:
1) the current state of awareness of pDPN among
physicians in the region,
2) key features of patient presentations with
pDPN,
3) issues and challenges facing physicians with
respect to screening for neuropathic pain,
4) goals and routine management strategies,
5) problems with initiating and maintaining
treatment, and
6) methods for optimizing the patient-physician
relationship based on evidence-based guidelines
on the screening, diagnosis and management of
pDPN.
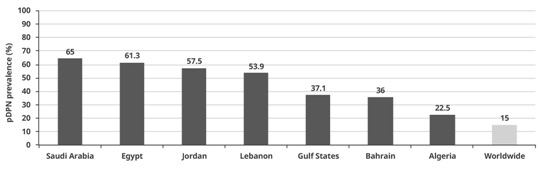
Figure 1: Estimated prevalence of pDPN among
patients with diabetes mellitus in countries
in the Middle East and North Africa
(1, 10, 13)
Epidemiology
Estimates
of
the
prevalence
of
pDPN
vary
widely
in
the
region
due
to
a
paucity
of
data.
Moreover,
current
prevalence
rates
may
be
underestimated
as
patients
often
do
not
approach
their
physicians
with
symptoms
of
neuropathic
pain
and
many
remain
undiagnosed.(8)
The
worldwide
prevalence
of
pDPN
among
patients
with
DM
is
estimated
to
be
around
30%,
however
in
the
Middle
East
and
North
Africa
this
ranges
from
22.5-65%
(Figure
1).
(1,
10,
13)
Of
note,
most
data
have
been
gathered
from
patients
in
secondary
and
tertiary
care
centers
and
studies
in
primary
care
are
required
to
establish
the
true
prevalence
of
pDPN.
Figure
2:
Intense
pain,
sleep
disturbance,
and
mood
disorders
significantly
reduce
the
quality
of
life
and
functionality
of
patients
with
pDPN
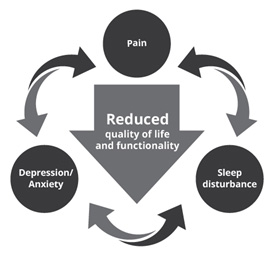
Burden
As
pDPN
can
have
significant
adverse
effects
on
quality
of
life,
early
recognition
of
neuropathic
symptoms
is
crucial.
Severe
pain
may
significantly
interfere
with
a
patient's
ability
to
exercise
or
walk,
limit
general
activities
of
daily
living,
and
alter
sleep
patterns
as
symptoms
are
often
most
intense
at
night(5,
14,
15).
Indeed,
one
study
reported
that
patients
with
pDPN
who
suffered
from
severe
pain
were
willing
to
trade
nearly
a
full
day
of
their
lives
in
order
to
avoid
1
additional
hour
of
pain.(15)
In
addition,
they
also
reported
feeling
approximately
(21)
years
older
than
their
actual
age.(15)
The
combination
of
intense
pain,
sleep
disturbance,
poor
mobility,
and
inability
to
perform
general
activities
of
daily
living
imposes
a
heavy
toll
on
patients,
which
are
strongly
associated
with
mood
disorders
such
as
depression
and
anxiety
(Figure
2).(16)
Whilst
a
previous
study
of
200
Emirati
subjects
with
diabetes
demonstrated
an
above
average
QoL
score,
this
was
significantly
reduced
in
those
with
at
least
one
complication
affecting
the
'foot',
heart,
eye
or
kidney.(17)
Etiology
and
risk
factors
The
exact
etiology
of
pDPN
is
unknown
and
may
be
attributable
to
a
combination
of
chronic
hyperglycemia
and
cardiovascular
risk
factors
such
as
dyslipidaemia
and
hypertension.(18)
The
Diabetes
Control
and
Complications
Trial
(DCCT)
and
its
follow-up
study,
the
Epidemiology
of
Diabetes
Interventions
and
Complications
(EDIC)
trial,
showed
that
intensive
glycemic
control
can
delay
the
development
and
progression
of
diabetic
neuropathy
in
patients
with
type
1
DM
(T1DM).(19,
20)
However,
patients
with
type
2
DM
(T2DM)
do
not
seem
to
benefit
from
intensive
glycemic
control
as
per
a
Cochrane
meta-analysis
and
several
large
clinical
trials.(4,
21,
22)
Lipid
levels,
blood
pressure,
inflammation,
insulin
resistance,
oxidative
stress,
vitamin
D
deficiency,
height,
cigarette
smoking,
and
alcohol
consumption
have
all
been
related
to
pDPN
in
various
studies.(2,
10,
22-24)
Epidemiological
analyses
suggest
that
the
following
are
significant
risk
factors
for
pDPN(25):
•
long-standing
diabetes
of
>10
years'
duration
•
age
>65
years
•
body
mass
index
(BMI)
>30
•
female
sex
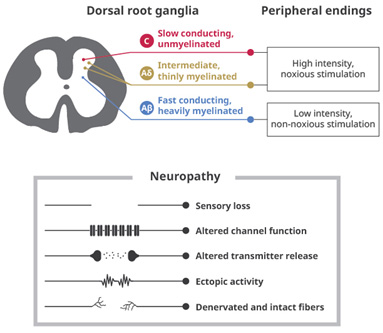
Pathophysiology
The
exact
mechanisms
underlying
pDPN
remain
unclear,
but
both
the
peripheral
and
central
nervous
system
are
thought
to
be
involved.
Painful
sensations
can
arise
from
damage
to
the
A
and
C
nerve
fibers.
Lesions
of
the
peripheral
nerves
can
increase
their
excitability,
a
phenomenon
known
as
peripheral
sensitization.
This
occurs
via
increased
or
altered
sodium
channel
expression
and
function,
which
is
associated
with
spontaneous
painful
discharges
and
reduced
thresholds
for
activation
leading
to
neuropathic
pain.(26)
Increased
calcium
channel
expression
also
encourages
the
release
of
excitatory
neurotransmitters
such
as
glutamate
and
substance
P,
and
can
increase
the
excitability
of
neurons
in
the
spinal
cord
(central
sensitization)(Figure
3).(23,
27)
Efferent
nerve
fibers
that
descend
from
the
brain
to
the
dorsal
horn
of
the
spinal
cord
have
a
powerful
inhibitory
effect
on
pain
signals
sent
via
the
afferent
fibres;
their
function
is
reliant
on
neurotransmitters
such
as
y-aminobutyric
acid
(GABA),
serotonin,
and
noradrenalin.
Dysfunction
of
these
pathways
can
also
result
in
central
sensitization.(27)
Figure
3.
Pain
pathways
in
patients
with
diabetic
peripheral
neuropathy
Clinical
presentation
Patients
with
pDPN
tend
to
describe
their
symptoms
using
similar
terms
and
most
describe
either
numbness
or
a
tingling,
electric
shock-like,
burning,
shooting
or
stabbing
pain
in
their
feet
or
lower
legs
due
to
the
involvement
of
the
small
sensory
nerve
fibers
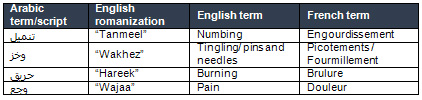
(Table
1).(5,
28,
29)
Table
1.
Common
Arabic,
English
and
French
terms
used
to
describe
symptoms
of
neuropathic
pain
by
patients
with
pDPN.
A
classic
'stocking
-
glove'
distribution
of
pain
is
expected
with
symptoms
initially
occurring
in
the
toes,
feet
and
lower
limbs
and
in
advanced
cases
progressing
to
the
fingers
and
hands.(4)
Many
patients
report
that
their
symptoms
are
worse
at
night.(5,
28,
29)
Practice
points:
Typical
symptoms
of
pDPN
Symptoms
vary
among
patients
but
typically
include
at
least
one
of
the
following:
•
Numbness
•
Tingling
sensation
•
Shooting/Stabbing
pain
•
Burning
sensation |
Other
characteristics
•
Worse
at
night
•
Stocking
-
glove
distribution:
Starts
in
the
feet,
progresses
up
the
lower
limbs.
Table
2:
Conditions
to
be
ruled
out
when
considering
a
pDPN
diagnosis
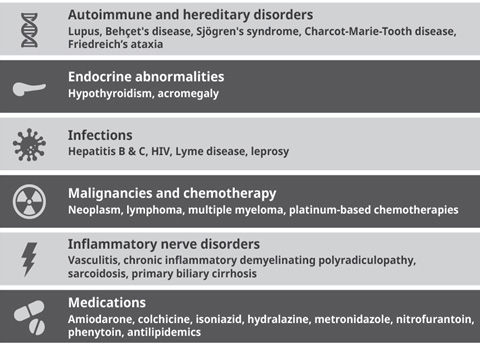
Diagnosis
and
evaluation
A
comprehensive
physical
examination
should
be
performed
when
a
patient
with
diabetes
presents
with
neuropathic
pain.
A
careful
clinical
history
should
be
undertaken
as
the
differential
diagnoses
are
many
and
varied,
and
diagnosis
may
be
challenging
(Table
2).
The
first
step
in
assessing
a
patient
is
to
determine
whether
there
is
any
evidence
of
neuropathic
pain.
Neuropathic
pain
can
be
distinguished
from
nociceptive
pain
by
using
a
screening
tool
and
performing
a
thorough
clinical
examination
(Table
3).
Table
3:
Tests
to
be
performed
before
diagnosing
pDPN
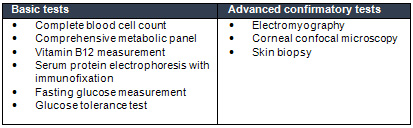
It
is
recommended
that
all
patients
with
metabolic
syndrome/impaired
glucose
tolerance
and
Type
1/2
diabetes
should
be
screened
for
neuropathic
pain
at
least
annually.
A
number
of
different
screening
tools
are
available
that
can
aid
with
differentiating
neuropathic
and
nociceptive
pain
including
the
Neuropathic
Pain
Questionnaire,
the
Leeds
Assessment
of
Neuropathic
Pain
and
Symptoms
scale
and
the
McGill
Pain
Questionnaire.(18,
25)
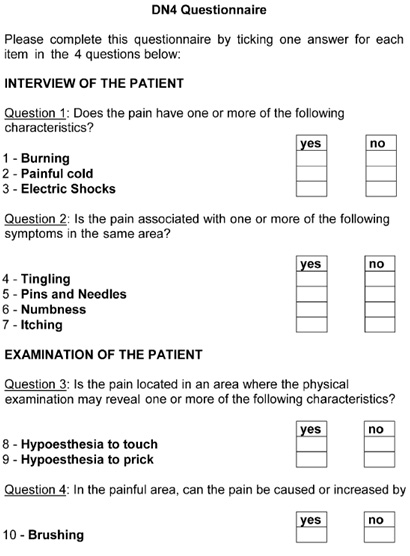
The
Doleur
Neuropathique
en
4
Questions
(DN4)
scale
is
recommended
as
it
has
a
sensitivity
and
specificity
of
82.9%
and
89.9%,
respectively,
and
validated
translations
are
available
in
15
languages,
including
English,
Arabic
and
French
(see:
Appendix).(25,
29,
30)
It
is
a
10-item
questionnaire
-
7
items
are
based
on
symptoms
and
3
on
a
simple
and
easy
to
perform
clinical
examination.
A
score
?4
is
suggestive
of
neuropathic
pain
(Figure
4).(25)
Figure
4:
English
translation
of
the
Doleur
Neuropathique
en
4
Questions
(DN4)
scale
recommended
for
distinguishing
nociceptive
and
neuropathic
pain
(29)
Patients
with
pDPN
present
initially
with
pain
in
the
feet
and
physicians
are
thus
advised
to
remove
a
patient's
shoes
and
socks
to
undertake
a
thorough
clinical
examination
of
the
patient's
feet.
Tests
include
not
only
the
three
tests
performed
as
part
of
the
DN4
(testing
for
touch,
pinprick,
and
allodynia),
but
also
a
more
careful
analysis
of
the
patient's
description
of
their
pain.
Traditionally,
a
monofilament
has
been
used
for
assessing
neuropathy;
however,
this
is
only
useful
for
evaluating
advanced
neuropathy
and
for
identifying
patients
at
high
risk
of
foot
ulceration.
It
should
therefore
not
be
used
to
identify
neuropathy
as
it
will
often
be
normal
despite
significant
small
fiber
neuropathy.
The
3L
(listen,
look,
locate)
approach
is
recommended
for
identifying
signs
and
symptoms
of
neuropathic
pain(27):
•
Listen
to
the
patient's
verbal
description
of
their
pain
and
note
any
mention
of
non-painful
symptoms
that
are
experienced
in
the
same
area
as
the
pain.
•
Look
for
any
sensory
abnormalities
such
as
pain
felt
upon
touching
the
sensitive
area
and
note
any
unusually
warm
or
cold
regions
and
differences
in
color
or
texture
relative
to
a
non-painful
adjacent
site.
•
Locate
the
region
of
pain
and
document
its
position
using
a
pain
drawing,
which
can
be
created
by
the
patient
or
the
physician.
Make
a
note
of
any
abnormal
sensations
on
the
drawing.
Practice
points:
Recommendations
for
Clinical
Assessment
•
Apply
the
DN4
screening
tool
to
identify
neuropathic
vs
nociceptive
pain
•
Remove
the
patient's
shoes
and
socks
and
examine
his/her
feet
•
Employ
the
3L
approach:
Listen
to
the
vocal
description
of
pain,
locate
the
region
of
pain,
and
look
for
somatosensory
deficits
with
the
help
of
simple
bedside
tests
|
Increasing
patient
awareness
is
important
for
encouraging
patients
to
self-report
painful
symptoms.
Patients
may
not
volunteer
this
information
and
it
is
recommended
that
physicians
ensure
that
all
at-risk
patients
are
aware
of
the
possibility
of
developing
pDPN
and
of
the
symptoms
they
should
look
out
for.
It
is
also
important
to
note
that
approximately
10-15%
of
patients
with
diabetes
who
experience
neuropathic
symptoms
will
have
a
neuropathy
from
another
cause
that
may
be
treatable.
Practice
points:
'Red
flags'
indicating
that
referral
to
a
neurologist
is
required
•
Asymmetrical
pain
•
Predominance
of
motor
vs
sensory
neuropathy
•
Rapid
progression
•
Acute
onset
•
Prominent
autonomic
symptoms |
Contemporary
Management
There
is
currently
no
cure
for
pDPN
and
treatment
is
challenging
as
many
patients
do
not
experience
sufficient
pain
relief.(28,
31)
In
this
context,
combination
pain
relief
regimens
may
be
more
effective
than
the
traditional
approach
of
trialing
and
discontinuing
a
single
agent
if
this
fails
to
provide
sufficient
relief.(31)
Treatment
goals
are
focused
on
preventing
the
development
or
progression
of
DPN
through
an
improvement
in
glycaemic
control
and
vascular
risk
factors
and
educating
patients
as
to
how
best
to
address
their
symptoms,
employing
pharmacologic
and/or
non-pharmacologic
agents
to
help
manage
neuropathic
pain
to
improve
the
patient's
QoL.(5)
Practice
points:
Treatment
Goals
•
A
clinically
meaningful
reduction
in
pain(32):
...o
30-50%
reduction
in
pain
on
a
visual
analog
scale
or
...o
2-point
reduction
on
a
10-point
Likert
scale
•
Manage
sleep
•
Educate
patients
..
o
Provide
information
on
pDPN
...o
Provide
guidance
on
self-care |
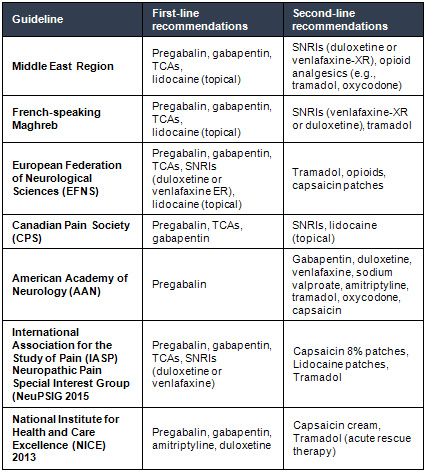
First-
and
second-line
recommendations
for
treatment
published
in
guidelines
for
the
Middle
East
and
North
Africa
are
consistent
with
those
used
globally
and
are
summarized
in
Table
4.
Most
recent
guidelines
for
treating
neuropathic
pain
-
including
the
International
Association
for
the
Study
of
Pain
Neuropathic
Pain
Special
Interest
Group
(NeuPSIG)
-
recommend
the
use
of
anticonvulsant
GABA
analogs
(pregabalin
or
gabapentin),
serotonin-norepinephrine
reuptake
inhibitors
(SNRIs
e.g.,
duloxetine),
or
tricyclic
antidepressants
(TCAs)
as
first-line
therapy.
Other
options
include
opioid
analgesics
(Table
5).(31,
32)
Other
therapies
such
as
topical
nitrate,
vitamin
D
and
alpha
lipoic
acid,
(33,
34)
as
well
as
non-pharmacological
therapy
such
as
acupuncture,
and
transcutaneous
electrical
nerve
stimulation
may
also
have
a
place
in
treating
patients
with
pDPN.
Vitamin
B
is
frequently
used
in
pDPN
management
in
the
MENA
region,
however,
there
are
limited
data
in
randomized
trials
testing
the
efficacy
of
vitamin
B
for
treating
peripheral
neuropathy
and
meta-analyses
have
reported
inconclusive
evidence
for
its
role
in
therapy.(35)
Table
4:
Global
first-
and
second-line
recommendations
for
the
treatment
of
neuropathic
pain
associated
with
diabetic
peripheral
neuropathy
(31,
32)
Practice
points:
Pharmacologic
therapy
of
pDPN
•
Use
GABA
analogs
(pregabalin
or
gabapentin),
tricyclic
antidepressants
(TCAs)
and
serotonin-norepinephrine
reuptake
inhibitors
(SNRIs)
first-line
•
Consider
combining
pregabalin
or
gabapentin
with
SNRIs
or
TCAs
•
Opioid
analgesics
can
be
given
for
second-line
use |
Patient
counseling
Patients
should
be
given
clear,
specific
information
on
how
to
use
their
medications
as
well
as
counseling
on
expectation
of
the
response
to
therapy,
as
an
expected
response
may
be
50%
at
best
whilst
a
30%
reduction
in
pain
is
considered
clinically
meaningful.
Patients
may
be
disappointed
if
they
expect
complete
resolution
of
their
painful
symptoms.
Physicians
should
clarify
that
the
agents
prescribed
are
used
for
a
specific
purpose
and
that
many
have
multiple
indications:
TCAs
for
example
are
prescribed
for
their
effect
on
neuropathic
pain
not
because
of
their
antidepressant
effects
and
this
should
be
made
clear
to
patients.
In
addition,
patients
should
also
be
educated
about
the
side
effects
of
different
medications
especially
when
prescribed
in
high
doses
and
both
benefits
and
disadvantages
should
be
openly
discussed
with
the
patient,
to
ensure
compliance.
It
is
also
important
to
follow-up
on
treatment
to
ensure
that
the
prescribed
medication
is
having
the
intended
effect;
if
not,
the
dose
should
be
increased
or
an
alternative
be
prescribed.
A
simple
11-point
pain
scale
can
be
useful
for
distinguishing
'before'
and
'after'
pain
scores
to
help
determine
the
effectiveness
of
the
prescribed
treatment.
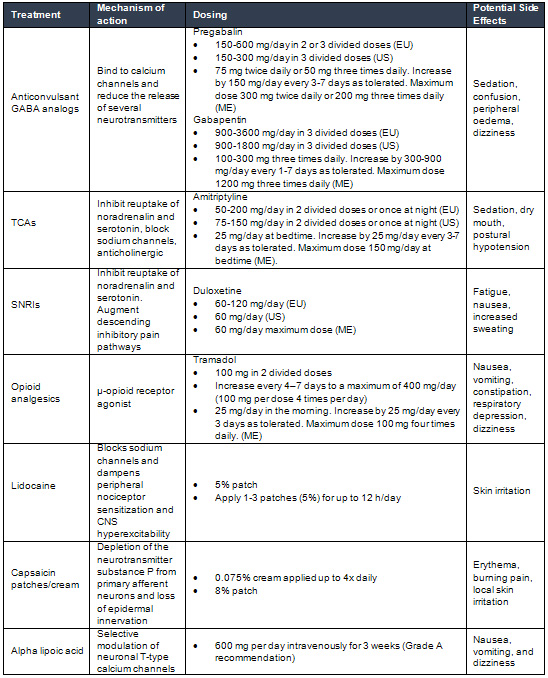
Table
5:
Summary
characteristics
of
treatments
recommended
for
neuropathic
pain
associated
with
diabetic
peripheral
neuropathy.29,
31,
32,
34,
36
EU:
European
Union;
ME:
Middle
East;
US:
United
States
pDPN
is
increasingly
common
in
the
Middle
East
and
North
Africa
but
is
underdiagnosed.
Physicians
in
the
region
are
encouraged
to
familiarize
themselves
with
the
latest
guidelines
for
diagnosing
and
managing
the
condition.
The
use
of
screening
tools
such
as
the
DN4
should
be
combined
with
a
comprehensive
physical
examination
to
identify
patients
suffering
from
pDPN
and
DPN.
The
3L
(listen,
look,
locate)
approach
to
clinical
assessment
is
recommended.
Physicians
should
educate
their
patients
on
identifying
pDPN
to
avoid
unnecessary
suffering
with
loss
of
sleep
and
a
reduced
QoL.
Once
neuropathy
has
been
diagnosed,
causes
other
than
pDPN
should
be
excluded.
Affected
patients
can
be
managed
according
to
international
treatment
recommendations
for
pDPN.
Anticonvulsants
(such
as
pregabalin
and
gabapentin),
SNRIs
and
TCAs
are
recommended
for
first-line
treatment.
Patients
should
be
closely
followed-up
and
those
with
inadequate
pain
relief
should
be
offered
an
alternative
first-line
agent
that
has
a
different
mechanism
of
action,
or
combination
therapy.
Second-line
agents
should
be
reserved
for
patients
who
suffer
more
severe
pain
and
who
are
unable
to
obtain
adequate
relief
from
first-line
agents
either
alone
or
in
combination.
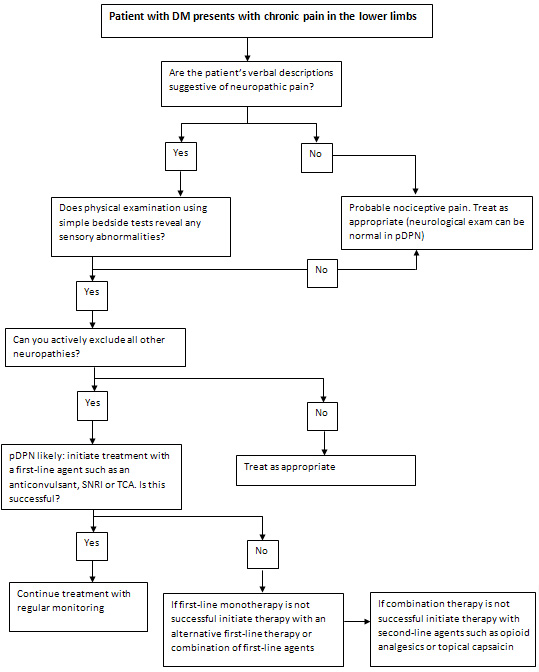
Treatment
Algorithm
Acknowledgements
Dr
Sid
Ahmed
Kherraf
of
Pfizer
Inc.
coordinated
the
expert
panel
meeting.
Editorial
and
writing
support
was
provided
by
Ms
Lianne
Cowie
and
Mr
Andy
Lee
of
MIMS
(Hong
Kong)
Limited
and
was
funded
by
Pfizer.
Pfizer
provided
an
unrestricted
educational
grant
but
did
not
write
or
control
the
scientific
content
of
the
manuscript.
Appendix
A
-
English
DN4
questionnaire
(29)

Appendix
B
-
French
DN4
questionnaire
(29)
Appendix
C
-
Arabic
DN4
questionnaire
(30)
1.
Halawa
MR,
Karawagh
A,
Zeidan
A,
Mahmoud
AE,
Sakr
M,
Hegazy
A.
Prevalence
of
painful
diabetic
peripheral
neuropathy
among
patients
suffering
from
diabetes
mellitus
in
Saudi
Arabia.
Curr
Med
Res
Opin.
2010
Feb;26(2):337-43.
2.
Boulton
AJ,
Vinik
AI,
Arezzo
JC,
Bril
V,
Feldman
EL,
Freeman
R,
et
al.
Diabetic
neuropathies:
a
statement
by
the
American
Diabetes
Association.
Diabetes
Care.
2005
Apr;28(4):956-62.
3.
Alamdari
A,
Mozafari
R,
Tafakhori
A,
Faghihi-Kashani
S,
Hafezi-Nejad
N,
Sheikhbahaei
S,
et
al.
An
inverse
association
between
serum
vitamin
D
levels
with
the
presence
and
severity
of
impaired
nerve
conduction
velocity
and
large
fiber
peripheral
neuropathy
in
diabetic
subjects.
Neurol
Sci.
2015
Jul;36(7):1121-6.
4.
Callaghan
BC,
Little
AA,
Feldman
EL,
Hughes
RA.
Enhanced
glucose
control
for
preventing
and
treating
diabetic
neuropathy.
Cochrane
Database
Syst
Rev.
2012
Jun
13(6):CD007543.
5.
Kuritzky
L,
Samraj
GP,
Argoff
CE.
Current
treatments
in
the
management
of
diabetic
peripheral
neuropathic
pain.
Pain
Med.
2009;63:62-73.
6.
Schumacher
C,
Glosner
SE.
Assessment
of
pain
and
impact
of
care
among
patients
with
painful
diabetic
peripheral
neuropathy.
J
Am
Pharm
Assoc
(2003).
2014
Jan-Feb;54(1):14-8.
7.
Boulton
AJ,
Gries
FA,
Jervell
JA.
Guidelines
for
the
diagnosis
and
outpatient
management
of
diabetic
peripheral
neuropathy.
Diabet
Med.
1998
Jun;15(6):508-14.
8.
Boulton
AJ.
Management
of
diabetic
peripheral
neuropathy.
Clinical
diabetes.
2005;23:9-15.
9.
Herman
WH,
Kennedy
L.
Underdiagnosis
of
peripheral
neuropathy
in
type
2
diabetes.
Diabetes
Care.
2005
Jun;28(6):1480-1.
10.
Jambart
S,
Ammache
Z,
Haddad
F,
Younes
A,
Hassoun
A,
Abdalla
K,
et
al.
Prevalence
of
painful
diabetic
peripheral
neuropathy
among
patients
with
diabetes
mellitus
in
the
Middle
East
region.
J
Int
Med
Res.
2011;39(2):366-77.
11.
Zabetian
A,
Keli
HM,
Echouffo-Tcheugui
JB,
Narayan
KM,
Ali
MK.
Diabetes
in
the
Middle
East
and
North
Africa.
Diabetes
Res
Clin
Pract.
2013
Aug;101(2):106-22.
12.
Petropoulos
IN,
Javed
S,
Azmi
S,
Khan
AN,
Malik
RA.
Diabetic
neuropathy
and
painful
diabetic
neuropathy
in
the
Middle
East
and
North
Africa
(MENA)
region:
Much
work
needs
to
be
done.
Journal
of
Taibah
University
Medical
Sciences.
2016;11(4):284-94.
13.
Aouiche
S,
Ouerdane
K,
Frioui
M,
Boudiba
A.
Painful
Diabetic
Neuropathy:
Frequency,
Risk
Factors,
and
Severity
in
a
Cohort
of
400
Diabetic
Patients
in
Algeria.
Médecine
des
Maladies
Métabolique.
2014;8(2):211-5.
14.
Quattrini
C,
Tesfaye
S.
Understanding
the
impact
of
painful
diabetic
neuropathy.
Diabetes
Metab
Res
Rev.
2003
Jan-Feb;19
Suppl
1:S2-8.
15.
Stacey
BR,
daCosta
DiBonaventura
M,
Martin
S.
Assessing
the
Relationship
between
Pain
Intensity
and
Quality
of
Life
in
Subjects
with
Painful
Diabetic
Peripheral
Neuropathy.
Poster
Presentation
#130
at
the
29th
Annual
Scientific
Meeting
of
the
American
Pain
Society;
Baltimore,
MD,
USA;
May
6-8,
2010.
16.
Tesfaye
S,
Boulton
AJ,
Dickenson
AH.
Mechanisms
and
management
of
diabetic
painful
distal
symmetrical
polyneuropathy.
Diabetes
Care.
2013
Sep;36(9):2456-65.
17.
Bani-Issa
W.
Evaluation
of
the
health-related
quality
of
life
of
Emirati
people
with
diabetes:
integration
of
sociodemographic
and
disease-related
variables.
East
Mediterr
Health
J.
2011
Nov;17(11):825-30.
18.
The
effect
of
intensive
treatment
of
diabetes
on
the
development
and
progression
of
long-term
complications
in
insulin-dependent
diabetes
mellitus.
The
Diabetes
Control
and
Complications
Trial
Research
Group.
N
Engl
J
Med.
1993
Sep
30;329(14):977-86.
19.
Albers
JW,
Herman
WH,
Pop-Busui
R,
Feldman
EL,
Martin
CL,
Cleary
PA,
et
al.
Effect
of
prior
intensive
insulin
treatment
during
the
Diabetes
Control
and
Complications
Trial
(DCCT)
on
peripheral
neuropathy
in
type
1
diabetes
during
the
Epidemiology
of
Diabetes
Interventions
and
Complications
(EDIC)
Study.
Diabetes
Care.
2010
May;33(5):1090-6.
20.
Gaede
P,
Lund-Andersen
H,
Parving
HH,
Pedersen
O.
Effect
of
a
multifactorial
intervention
on
mortality
in
type
2
diabetes.
N
Engl
J
Med.
2008
Feb
7;358(6):580-91.
21.
Ismail-Beigi
F,
Craven
T,
Banerji
MA,
Basile
J,
Calles
J,
Cohen
RM,
et
al.
Effect
of
intensive
treatment
of
hyperglycaemia
on
microvascular
outcomes
in
type
2
diabetes:
an
analysis
of
the
ACCORD
randomised
trial.
Lancet.
2010
Aug
7;376(9739):419-30.
22.
Davis
TM,
Yeap
BB,
Davis
WA,
Bruce
DG.
Lipid-lowering
therapy
and
peripheral
sensory
neuropathy
in
type
2
diabetes:
the
Fremantle
Diabetes
Study.
Diabetologia.
2008
Apr;51(4):562-6.
23.
Celikbilek
A,
Gocmen
AY,
Tanik
N,
Borekci
E,
Adam
M,
Celikbilek
M,
et
al.
Decreased
serum
vitamin
D
levels
are
associated
with
diabetic
peripheral
neuropathy
in
a
rural
area
of
Turkey.
Acta
Neurol
Belg.
2015
Mar;115(1):47-52.
24.
Wiggin
TD,
Sullivan
KA,
Pop-Busui
R,
Amato
A,
Sima
AA,
Feldman
EL.
Elevated
triglycerides
correlate
with
progression
of
diabetic
neuropathy.
Diabetes.
2009
Jul;58(7):1634-40.
25.
Chetty
S,
Baalbergen
E,
Bhigjee
AI,
Kamerman
P,
Ouma
J,
Raath
R,
et
al.
Clinical
practice
guidelines
for
management
of
neuropathic
pain:
expert
panel
recommendations
for
South
Africa.
S
Afr
Med
J.
2012
Mar
08;102(5):312-25.
26.
Lauria
G,
Ziegler
D,
Malik
R,
Merkies
IS,
Waxman
SG,
Faber
CG.
The
role
of
sodium
channels
in
painful
diabetic
and
idiopathic
neuropathy.
Curr
Diab
Rep.
2014
Oct;14(10):538.
27.
Gilron
I,
Watson
CP,
Cahill
CM,
Moulin
DE.
Neuropathic
pain:
a
practical
guide
for
the
clinician.
CMAJ.
2006
Aug
1;175(3):265-75.
28.
Baron
R,
Binder
A,
Wasner
G.
Neuropathic
pain:
diagnosis,
pathophysiological
mechanisms,
and
treatment.
Lancet
Neurol.
2010
Aug;9(8):807-19.
29.
Bouhassira
D,
Attal
N,
Alchaar
H,
Boureau
F,
Brochet
B,
Bruxelle
J,
et
al.
Comparison
of
pain
syndromes
associated
with
nervous
or
somatic
lesions
and
development
of
a
new
neuropathic
pain
diagnostic
questionnaire
(DN4).
Pain.
2005
Mar;114(1-2):29-36.
30.
Harifi
G,
Ouilki
I,
El
Bouchti
I,
Ouazar
MA,
Belkhou
A,
Younsi
R,
et
al.
Validity
and
reliability
of
the
Arabic
adapted
version
of
the
DN4
questionnaire
(Douleur
Neuropathique
4
Questions)
for
differential
diagnosis
of
pain
syndromes
with
a
neuropathic
or
somatic
component.
Pain
Pract.
2011
Mar-Apr;11(2):139-47.
31.
Javed
S,
Alam
U,
Malik
RA.
Burning
through
the
pain:
treatments
for
diabetic
neuropathy.
Diabetes
Obes
Metab.
2015
Dec;17(12):1115-25.
32.
Ziegler
D,
Fonseca
V.
From
guideline
to
patient:
a
review
of
recent
recommendations
for
pharmacotherapy
of
painful
diabetic
neuropathy.
J
Diabetes
Complications.
2015
Jan-Feb;29(1):146-56.
33.
Basit
A,
Basit
KA,
Fawwad
A,
Shaheen
F,
Fatima
N,
Petropoulos
IN,
et
al.
Vitamin
D
for
the
treatment
of
painful
diabetic
neuropathy.
BMJ
Open
Diabetes
Res
Care.
2016;4(1):e000148.
34.
Mijnhout
GS,
Kollen
BJ,
Alkhalaf
A,
Kleefstra
N,
Bilo
HJ.
Alpha
lipoic
Acid
for
symptomatic
peripheral
neuropathy
in
patients
with
diabetes:
a
meta-analysis
of
randomized
controlled
trials.
Int
J
Endocrinol.
2012;2012:456279.
35.
Ang
CD,
Alviar
MJ,
Dans
AL,
Bautista-Velez
GG,
Villaruz-Sulit
MV,
Tan
JJ,
et
al.
Vitamin
B
for
treating
peripheral
neuropathy.
Cochrane
Database
Syst
Rev.
2008
Jul
16(3):CD004573.
36.
Bohlega
S,
Alsaadi
T,
Amir
A,
Hosny
H,
Karawagh
AM,
Moulin
D,
et
al.
Guidelines
for
the
pharmacological
treatment
of
peripheral
neuropathic
pain:
expert
panel
recommendations
for
the
Middle
East
region.
J
Int
Med
Res.
2010
Mar-Apr;38(2):295-317.
|
|
.................................................................................................................

|
| |
|

