|
|
 |
| ............................................................. |
|
|
| ........................................................ |
| From
the Editor |

|
Editorial
A. Abyad (Chief Editor) |
|
|
|
|
........................................................
|
Original
Contribution/Clinical Investigation
|
|

|
<-- Turkey -->
Preoperative
management of sickle cell patients with hydroxyurea
[pdf version]
Mehmet Rami Helvaci,
Sedat Hakimoglu, Mehmet Oktay Sariosmanoglu,
Suleyman Kardas, Beray Bahar, Merve Filoglu,
Ibrahim Ugur Deler, Duygu Alime Almali, Ozcan
Gokpinar, Ozlem Celik, Aynur Ozbay, Ozgun Ilke
Karagoz, Seher Aydin
<-- Ethiopia-->
Khat
(Catha edulis) chewing as a risk factor of low
birth weight among full term Newborns: A systematic
review
[pdf version]
Kalkidan Hassen
<-- Australia -->
Chronic
pain review following Lichtenstein hernia repair:
A Personal Series
[pdf
version]
Maurice Brygel,
Luke Bonato, Sam Farah
<-- Saudi Arabia -->
Assessment
of Health Status of Male Teachers in Abha City,
Saudi Arabia
[pdf
version]
Ali Mofareh Assiri,
Hassan M. A. Al-Musa
|
|
........................................................ |
Evidence
Based Medicine
........................................................
Medicine and Society
........................................................
|
Chief
Editor -
Abdulrazak
Abyad
MD, MPH, MBA, AGSF, AFCHSE
.........................................................
Editorial
Office -
Abyad Medical Center & Middle East Longevity
Institute
Azmi Street, Abdo Center,
PO BOX 618
Tripoli, Lebanon
Phone: (961) 6-443684
Fax: (961) 6-443685
Email:
aabyad@cyberia.net.lb
.........................................................
Publisher
-
Lesley
Pocock
medi+WORLD International
11 Colston Avenue,
Sherbrooke 3789
AUSTRALIA
Phone: +61 (3) 9005 9847
Fax: +61 (3) 9012 5857
Email:
lesleypocock@mediworld.com.au
.........................................................
Editorial
Enquiries -
abyad@cyberia.net.lb
.........................................................
Advertising
Enquiries -
lesleypocock@mediworld.com.au
.........................................................
While all
efforts have been made to ensure the accuracy
of the information in this journal, opinions
expressed are those of the authors and do not
necessarily reflect the views of The Publishers,
Editor or the Editorial Board. The publishers,
Editor and Editorial Board cannot be held responsible
for errors or any consequences arising from
the use of information contained in this journal;
or the views and opinions expressed. Publication
of any advertisements does not constitute any
endorsement by the Publishers and Editors of
the product advertised.
The contents
of this journal are copyright. Apart from any
fair dealing for purposes of private study,
research, criticism or review, as permitted
under the Australian Copyright Act, no part
of this program may be reproduced without the
permission of the publisher.
|
|
|
| October 2015 -
Volume 13 Issue 7 |
|
|
Preoperative management
of sickle cell patients with hydroxyurea
Mehmet
Rami Helvaci (1)
Sedat Hakimoglu (2)
Mehmet Oktay Sariosmanoglu (1)
Suleyman Kardas (1)
Beray Bahar (1)
Merve Filoglu (1)
Ibrahim Ugur Deler (1)
Duygu Alime Almali (1)
Ozcan Gokpinar (1)
Ozlem Celik (1)
Aynur Ozbay (1)
Ozgun Ilke Karagoz (1)
Seher Aydin (1)
(1) Medical Faculty of the Mustafa Kemal University,
Department of Internal Medicine, M.D.
(2) Medical Faculty of the Mustafa Kemal University,
Department of Anesthesiology and Reanimation,
M.D.
Correspondence:
Mehmet Rami Helvaci, M.D.
Medical Faculty of the Mustafa Kemal University
31100, Serinyol, Antakya, Hatay, TURKEY
Phone: 00-90-326-2291000 (Internal 3399)
Fax: 00-90-326-2455654
Email: mramihelvaci@hotmail.com
|
Abstract
Background:
We tried to understand whether or not
there are some beneficial changes of health
parameters with hydroxyurea in sickle
cell diseases (SCDs) cases.
Methods: All
SCDs cases were enrolled, and a hydroxyurea
therapy was initiated.
Results: We
studied 337 patients, totally. Hydroxyurea
was well-tolerated with a majority of
patients (80.1%). Mean number (10.3 versus
1.7 crises per year, p<0.000) and mean
severity of painful crises decreased,
significantly (7.8 versus 2.2, p<0.001).
Although body weight and mean hematocrit
(Hct) value increased, white blood cell
(WBC) and platelet (PLT) counts and total
and direct bilirubin and lactate dehydrogenase
(LDH) levels decreased, significantly
(p<0.000 for all). On the other hand,
there were avascular necrosis of bones
in 18.9%, leg ulcers in 12.7%, pulmonary
hypertension in 11.5%, chronic renal disease
in 8.3%, coronary heart disease in 7.7%,
digital clubbing in 6.5%, stroke in 6.5%,
exitus in 5.3%, chronic obstructive pulmonary
disease in 4.7%, and cirrhosis in 3.2%
of the patients.
Conclusion: SCDs
are chronic inflammatory disorders initiating
at birth. Hydroxyurea decreases frequency
and severity of painful crises, WBC and
PLT counts, and total and direct bilirubin
and LDH levels, and it increases body
weight and Hct value, all of which indicate
a decreased inflammatory process in patients.
Thus elective surgical procedures should
be performed after a few months of treatment
with hydroxyurea in non-users. By this
way, beside decreased requirement of blood
transfusions, perioperative morbidity
and mortality will also be lowered due
to decreased inflammatory process on capillary
endothelium all over the body.
Key words: Sickle
cell diseases, chronic endothelial inflammation,
hydroxyurea
|
Systemic atherosclerosis may be the major underlying
cause of aging in human beings and even in animals.
It is an irreversible process initiating at
birth. Although it keeps to mainly the larger,
high blood pressure (BP) carrying vessels, all
arteries, arterioles, and even capillaries are
affected with some extent. Some of the accelerating
factors of the systemic process are overweight,
dyslipidemia, elevated BP, and insulin resistance
for the development of terminal diseases such
as obesity, hypertension (HT), diabetes mellitus
(DM), coronary heart disease (CHD), chronic
obstructive pulmonary disease (COPD), cirrhosis,
chronic renal disease (CRD), peripheric artery
disease, and stroke, all of which are collected
under the heading of metabolic syndrome (1-6).
On the other hand, sickle cell diseases (SCDs)
are systemic microangiopathic processes that
are caused by homozygous inheritance of hemoglobin
S (Hb S) (7,8). Glutamic acid is replaced with
valine in the sixth position of the beta chain
of the Hb S. Presence of valine promotes polymerisation
of the Hb S. So Hb S causes red blood cells
(RBCs) to change their normal elastic and biconcave
disc shaped structures to hard bodies. The decreased
elasticity of RBCs instead of their shapes may
be the central pathology of the diseases. The
sickling process is present in whole life, but
is exaggerated during stressful conditions due
to the increased basal metabolic rate. The RBCs
can take their normal elastic structures after
normalization of the stressful conditions, but
after repeated cycles of sickling and unsickling,
they become hard bodies, permanently. The hard
cells induced chronic endothelial damage and
infarcts at the microvascular level, even in
the absence of obvious vascular occlusions due
to the edematous endothelium, are the terminal
consequences of the diseases, so life expectancy
is decreased up to 30 years (9). We tried to
understand whether or not there are some beneficial
changes of health parameters with hydroxyurea
therapy in the SCDs.
The
study
was
performed
in
the
Hematology
Service
of
the
Mustafa
Kemal
University
between
March
2007
and
October
2013.
All
patients
with
SCDs
were
enrolled
into
the
study.
SCDs
are
diagnosed
by
the
hemoglobin
electrophoresis
performed
via
high
performance
liquid
chromatography.
Their
medical
histories
including
smoking
habit,
regular
alcohol
consumption,
and
leg
ulcers
were
learnt.
Frequency
of
painful
crises
was
detected
as
a
mean
number
of
crises
per
year,
and
severity
of
them
as
a
mean
degree
between
0
to
10
according
to
patient's
self-explanation.
Cases
with
a
history
of
three
pack-year
were
accepted
as
smokers,
and
cases
with
a
history
of
one
drink
a
day
for
three
years
were
accepted
as
drinkers.
A
check
up
procedure
including
body
weight,
serum
creatinine
value
on
three
occasions,
hepatic
function
tests,
markers
of
hepatitis
viruses
A,
B,
and
C
and
human
immunodeficiency
virus,
an
electrocardiography,
a
Doppler
echocardiography,
an
abdominal
ultrasonography,
a
computed
tomography
of
brain,
and
a
magnetic
resonance
imaging
of
hips
was
performed.
Other
bone
areas
for
avascular
necrosis
were
scanned
according
to
the
patients'
complaints.
Cases
with
acute
painful
crisis
or
any
other
inflammatory
event
were
treated
at
first,
and
then
the
spirometric
pulmonary
function
tests
to
diagnose
COPD,
the
Doppler
echocardiography
to
measure
the
systolic
BP
of
pulmonary
artery,
and
renal
and
hepatic
function
tests
were
performed
on
the
silent
phase.
The
criterion
for
diagnosis
of
COPD
is
post-bronchodilator
forced
expiratory
volume
in
1
second/forced
vital
capacity
of
less
than
70%
(10).
Systolic
BP
of
the
pulmonary
artery
of
40
mmHg
or
higher
during
the
silent
phase
is
accepted
as
pulmonary
hypertension
(11).
CRD
is
diagnosed
with
a
permanently
elevated
serum
creatinine
level
of
1.3
mg/dL
or
higher
on
the
silent
phase.
Cirrhosis
is
diagnosed
with
hepatic
function
tests,
ultrasonographic
findings,
ascites,
and
liver
biopsy
in
case
of
requirement.
Digital
clubbing
is
diagnosed
with
the
ratio
of
distal
phalangeal
diameter
to
interphalangeal
diameter
of
greater
than
1.0
and
with
the
presence
of
Schamroth's
sign
(12,13).
A
stress
electrocardiography
was
performed
in
cases
with
an
abnormal
electrocardiography
and/or
angina
pectoris.
A
coronary
angiography
was
obtained
just
for
the
stress
electrocardiography
positive
cases.
So
CHD
was
diagnosed
either
angiographically
or
with
the
Doppler
echocardiographic
findings
as
the
movement
disorders
of
the
cardiac
walls.
Then,
a
hydroxyurea
therapy
was
initiated
to
all
patients
with
an
initial
dose
of
15
mg/kg/day,
and
then
the
dose
was
increased
up
to
the
final
dose
of
35
mg/kg/day
according
to
patients'
requirement
and
compliance.
Finally,
the
mean
number
and
severity
of
painful
crises,
body
weight,
white
blood
cell
(WBC)
and
platelet
(PLT)
counts,
hematocrit
(Hct)
value,
mean
corpuscular
volume
(MCV),
and
the
total
and
direct
bilirubin
and
lactate
dehydrogenase
(LDH)
levels
of
the
serum
were
compared
before
and
after
the
hydroxyurea
therapy.
Mann-Whitney
U
test,
Independent-Samples
t
test,
and
comparison
of
proportions
were
used
as
the
methods
of
statistical
analyses.
Table
1:
Characteristic
features
of
sickle
cell
patients
before
and
after
hydroxyurea
therapy
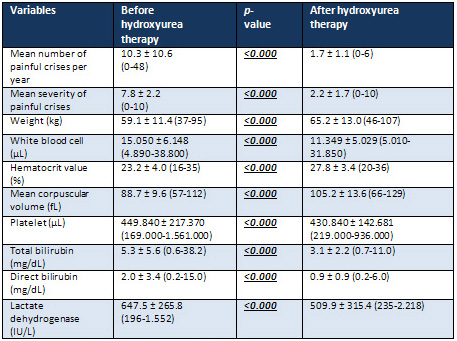
Table
2:
Sickle
cell
patients
with
associated
disorders
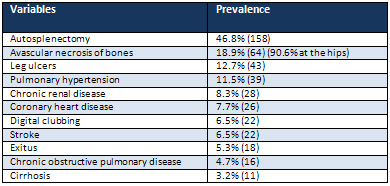
The
study
included
337
patients
with
the
SCDs
(169
females
and
168
males).
The
mean
ages
of
them
were
28.4
±
9.3
(8-59)
versus
29.8
±
9.3
(6-58)
years
in
females
and
males,
respectively
(p>0.05).
The
hydroxyurea
treatment
was
used
and
well-tolerated
with
a
high
majority
of
cases
(80.1%),
and
the
remaining
cases
could
not
be
followed
up.
We
have
not
observed
any
major
side
effect
of
the
therapy
during
the
follow-up
period.
The
final
dose
of
35
mg/kg/day
was
required
just
in
25
cases
(7.4%),
and
the
usual
dose
was
500
mg
twice
daily
during
the
7-year
follow-up
period.
During
the
period,
the
mean
number
of
painful
crises
per
year
was
significantly
decreased
with
the
treatment
(10.3
versus
1.7
crises
per
year,
p<0.000).
The
mean
severity
of
painful
crises
was
decreased,
too
(7.8
versus
2.2,
p<0.001).
Although
the
body
weight,
mean
Hct
value,
and
MCV
increased,
the
WBC
and
PLT
counts
and
the
total
and
direct
bilirubin
and
LDH
levels
of
the
serum
decreased
with
the
therapy,
significantly
(p<0.000
for
all)
(Table
1).
On
the
other
hand,
we
detected
autosplenectomy
in
46.8%,
avascular
necrosis
of
bones
in
18.9%
(90.6%
at
hips,
10.9%
at
shoulders,
9.3%
at
knees,
6.2%
at
elbows,
3.1%
at
ankles,
and
1.5%
at
wrists),
leg
ulcers
in
12.7%,
pulmonary
hypertension
in
11.5%,
CRD
in
8.3%,
CHD
in
7.7%,
digital
clubbing
in
6.5%,
stroke
in
6.5%,
exitus
in
5.3%,
COPD
in
4.7%,
and
cirrhosis
in
3.2%
of
the
patients
(Table
2).
Although
smoking
was
observed
in
6.5%
(22)
of
the
patients,
there
was
only
one
case
(0.2%)
of
regular
alcohol
consumption,
who
was
not
cirrhotic
at
the
time.
Although
antiHCV
was
positive
in
two
of
the
cirrhotics,
HCV
RNA
was
detected
as
negative
by
polymerase
chain
reaction
in
both.
Prevalences
of
mortality
were
similar
in
both
genders
(4.7%
versus
5.9%
in
females
and
males,
respectively,
p>0.05),
and
mean
ages
of
such
cases
were
32.1
versus
29.1
years
in
females
and
males,
respectively
(p>0.05).
SCDs
mainly
affect
microvascular
endothelium
of
the
body
(14),
because
the
capillary
system
is
the
main
distributor
of
the
hard
bodies
to
tissues,
so
it
is
destroyed
much
more
severely
than
the
larger
vessels.
Because
of
the
microvascular
nature
of
the
diseases,
we
can
observe
healing
of
leg
ulcers
with
hydroxyurea
therapy
in
early
years
of
life,
but
the
healing
process
is
difficult
due
to
the
excessive
fibrosis
around
the
capillaries
later
in
life.
Eventually,
the
mean
survival
was
42
years
in
males
and
48
years
in
females
in
the
literature
(9),
whereas
it
was
29
and
32
years,
respectively,
in
the
present
study
(p>0.05).
According
to
our
experiences,
the
great
differences
between
the
survival
are
secondary
to
the
initiation
of
hydroxyurea
treatment
in
early
years
of
life,
even
at
birth
in
developed
countries.
On
the
other
hand,
the
prolonged
survival
of
females
with
SCDs
and
the
longer
overall
survival
of
females
in
the
world
(15)
could
not
be
explained
by
well
known
strong
atherosclerotic
effects
of
smoking
alone;
instead
it
may
be
explained
by
the
dominant
role
of
male
sex
in
life
(16).
As
a
result
of
such
a
great
variety
of
clinical
presentations,
it
is
not
surprising
to
see
that
the
mean
body
weight
and
body
mass
index
(BMI)
were
retarded
in
the
SCDs
cases
(17).
Parallel
to
the
lower
body
weight
and
BMI,
the
low
density
lipoprotein
cholesterol,
alanine
aminotransferase,
and
systolic
and
diastolic
BPs
were
also
lower
in
the
SCDs
(17),
which
can
be
explained
by
definition
of
the
metabolic
syndrome
(18,19).
Painful
crises
are
the
pathognomonic
symptoms
of
the
SCDs.
Although
painful
crises
themselves
may
not
be
life
threatening
directly
(20),
increased
basal
metabolic
rate
with
any
underlying
cause
such
as
infection,
tissue
damage,
operation,
or
depression
usually
terminate
with
crises,
so
multiorgan
failures
on
the
chronic
inflammatory
background
of
the
SCDs
are
not
rare
in
such
circumstances
(21,22).
Probably
pain
is
due
to
the
disseminated
inflammatory
process
of
the
capillary
endothelium,
and
the
increased
WBC
and
PLT
counts
and
decreased
Hct
values
indicate
presence
of
a
chronic
inflammatory
process
during
their
whole
lives
in
such
patients.
Increased
WBC
counts
even
in
the
absence
of
an
infection,
tissue
damage,
operation,
or
depression
was
an
independent
predictor
of
the
disease
severity
(23),
and
it
was
associated
with
an
increased
risk
of
stroke,
probably
by
releasing
cytotoxic
enzymes
and
causing
endothelial
damage
in
the
brain
(24).
Due
to
the
severity
of
pain,
narcotic
analgesics
are
usually
required
to
control
them
(25),
but
according
to
our
practice,
simple
RBC
transfusions
are
highly
effective
during
the
severe
crises,
both
to
relieve
pain
and
to
prevent
sudden
death
that
may
develop
secondary
to
the
multiorgan
failures
on
the
prolonged
inflammatory
background
of
the
SCDs.
Hydroxyurea
is
an
effective
drug
in
several
chronic
myeloproliferative
disorders
and
SCDs.
It
interferes
with
cell
division
by
blocking
the
formation
of
deoxyribonucleotides
via
inhibition
of
ribonucleotide
reductase.
The
deoxyribonucleotides
are
building
blocks
of
DNA.
Hydroxyurea
mainly
acts
on
hyperproliferative
cells.
Although
the
action
of
hydroxyurea
is
thought
to
be
the
increase
of
gamma
globin
synthesis
for
fetal
hemoglobin
(Hb
F)
(26,27),
its
main
action
is
probably
suppression
of
leukocytosis
and
thrombocytosis
in
the
SCDs.
By
this
way,
the
continuous
inflammatory
process
of
the
SCDs
that
initiated
at
birth
on
the
capillary
endothelium
is
suppressed
with
some
extent.
Due
to
the
same
action
way,
hydroxyurea
is
also
used
in
moderate
and
severe
psoriasis
to
suppress
hyperproliferative
skin
cells.
As
in
viral
hepatitis
cases,
although
presence
of
a
continuous
damage
of
hard
RBCs
on
the
capillary
endothelium
in
the
SCDs,
the
severity
of
destructive
process
is
probably
exaggerated
by
the
patients'
immune
system,
especially
by
the
actions
of
WBCs
and
PLTs
(28).
So
suppression
of
excessive
proliferation
of
WBCs
and
PLTs
probably
limits
the
capillary
damage-induced
tissue
ischemia
and
infarctions
all
over
the
body.
Similarly,
it
was
reported
that
lower
neutrophil
counts
were
associated
with
lower
crises
rates,
and
if
a
tissue
infarction
occurs,
lower
neutrophil
counts
may
limit
severity
of
pain
and
extent
of
tissue
damage
(29).
On
the
other
hand,
final
Hb
F
levels
in
hydroxyurea
users
did
not
differ
from
their
pretreatment
levels,
significantly
(29).
Physicians
at
the
National
Institutes
of
Health
Consensus
Conference
agreed
that
hydroxyurea
is
underused
both
in
children
and
adults.
First
of
all,
due
to
the
relatively
younger
mean
ages
of
the
SCDs
patients,
females
and
even
males
may
not
use
the
drug
for
a
long
period
of
time
just
to
get
a
baby
with
some
additional
inhibitory
effects
of
the
chronic
inflammatory
disease
on
fertility.
Additionally,
there
is
fear
of
cancers
in
people,
since
hydroxyurea
is
a
chemotherapeutic
agent
(30).
However,
the
cancer
risk
has
not
been
substantiated
by
more
than
a
decade
of
using
hydroxyurea
for
adults
(31).
Although
some
data
show
risk
to
fetus
(32),
potential
benefits
may
outweigh
potential
risk
even
during
pregnancy.
According
to
our
experiences,
there
are
several
female
patients
with
infertility,
abortus,
and
stillbirth
in
the
absence
of
hydroxyurea
therapy,
and
the
decreased
number
and
severity
of
painful
crises,
increased
body
weight,
decreased
WBC
and
PLT
counts,
and
increased
Hct
value
with
the
hydroxyurea
therapy
will
probably
result
with
resolution
of
the
above
problems
to
some
extent.
It
is
clear
that
there
is
a
need
for
more
effective
treatment
regimens
in
the
SCDs,
but
until
they
become
more
available,
hydroxyurea
should
be
used
in
all
cases,
and
its
dose
should
be
kept
higher
in
the
moderate
and
severe
patients.
Hydroxyurea
may
have
a
critical
role
in
the
SCDs
(14).
The
Multicenter
Study
of
Hydroxyurea
(MSH)
studied
299
severely
affected
adults
with
sickle
cell
anemia
(Hb
SS),
and
compared
the
results
of
patients
treated
with
hydroxyurea
or
placebo
(33).
The
study
especially
searched
effects
of
the
drug
on
painful
crises,
acute
chest
syndrome,
and
need
of
RBC
transfusions.
The
outcomes
were
so
overwhelming
in
favour
of
hydroxyurea
that
the
study
was
terminated
after
22
months,
and
hydroxyurea
was
initiated
to
all
patients.
The
patients
treated
with
hydroxyurea
had
a
44%
decrease
of
hospitalizations,
and
there
was
a
strong
and
independent
association
of
lower
neutrophil
counts
with
the
lower
crisis
rates
(33).
But
this
study
was
performed
just
in
severe
Hb
SS
cases
alone,
and
the
rate
of
painful
crises
was
decreased
from
4.5
to
2.5
per
year
(33).
Whereas
in
our
study,
we
used
337
patients
with
all
subtypes
and
clinical
severity
of
SCDs,
and
the
rate
of
painful
crises
was
decreased
from
10.3
to
1.7
per
year
(p<0.000)
with
an
additional
decreased
severity
of
them
(7.8
versus
2.2,
p<0.000).
Parallel
to
the
above
results,
adult
SCDs
patients
using
hydroxyurea
appear
to
have
reduced
mortality
rate
after
a
9-year
follow-up
period
(34).
Although
the
underlying
disease
severity
remains
critical
to
determine
prognosis,
hydroxyurea
may
decrease
severity
of
disease
(34)
and
prolong
survival
(14).
Probably
chronic
endothelial
damage
of
the
capillaries
is
initiated
at
birth,
and
complications
may
start
to
be
seen
even
in
infancy.
For
example,
infants
with
lower
hemoglobin
levels
were
more
likely
to
have
higher
incidences
of
acute
chest
syndrome,
painful
crises,
and
lower
neuropsychological
scores,
and
hydroxyurea
reduced
the
incidence
of
them
(35).
Hydroxyurea
in
early
life
may
also
protect
splenic
function,
improve
growth,
and
prevent
multiorgan
dysfunctions
by
preventing
early
capillary
damage.
Transfusion
programmes
also
reduce
the
complications,
but
they
carry
risks
including
transmission
of
infections,
development
of
allo-antibodies
causing
subsequent
transfusions
difficult,
and
iron
overload.
As
a
conclusion,
the
SCDs
are
chronic
inflammatory
disorders
initiating
at
birth.
Hydroxyurea
decreases
frequency
and
severity
of
painful
crises,
WBC
and
PLT
counts,
and
total
and
direct
bilirubin
and
LDH
levels,
and
it
increases
body
weight
and
Hct
value,
all
of
which
indicate
a
decreased
inflammatory
process
in
the
patients.
Thus
elective
surgical
procedures
should
be
performed
after
a
few
months
of
treatment
with
hydroxyurea
in
non-users.
By
this
way,
beside
decreased
requirement
of
blood
transfusions,
perioperative
morbidity
and
mortality
will
also
be
lowered
due
to
decreased
inflammatory
process
on
capillary
endothelium
all
over
the
body.
1.
Eckel
RH,
Grundy
SM,
Zimmet
PZ.
The
metabolic
syndrome.
Lancet
2005;
365:
1415-1428.
2.
Helvaci
MR,
Kaya
H,
Gundogdu
M.
Association
of
increased
triglyceride
levels
in
metabolic
syndrome
with
coronary
artery
disease.
Pak
J
Med
Sci
2010;
26:
667-672.
3.
Helvaci
MR,
Kaya
H,
Borazan
A,
Ozer
C,
Seyhanli
M,
Yalcin
A.
Metformin
and
parameters
of
physical
health.
Intern
Med
2008;
47:
697-703.
4.
Helvaci
MR,
Kaya
H,
Seyhanli
M,
Yalcin
A.
White
coat
hypertension
in
definition
of
metabolic
syndrome.
Int
Heart
J
2008;
49:
449-457.
5.
Helvaci
MR,
Kaya
H,
Seyhanli
M,
Cosar
E.
White
coat
hypertension
is
associated
with
a
greater
all-cause
mortality.
J
Health
Sci
2007;
53:
156-160.
6.
Helvaci
MR,
Aydin
LY,
Aydin
Y.
Chronic
obstructive
pulmonary
disease
may
be
one
of
the
terminal
end
points
of
metabolic
syndrome.
Pak
J
Med
Sci
2012;
28:
376-379.
7.
Helvaci
MR,
Erden
ES,
Aydin
LY.
Atherosclerotic
background
of
chronic
obstructive
pulmonary
disease
in
sickle
cell
patients.
HealthMED
2013;
7:
484-488.
8.
Helvaci
MR,
Aydin
Y,
Ayyildiz
O.
Clinical
severity
of
sickle
cell
anemia
alone
and
sickle
cell
diseases
with
thalassemias.
HealthMED
2013;
7:
2028-2033.
9.
Platt
OS,
Brambilla
DJ,
Rosse
WF,
Milner
PF,
Castro
O,
Steinberg
MH,
et
al.
Mortality
in
sickle
cell
disease.
Life
expectancy
and
risk
factors
for
early
death.
N
Engl
J
Med
1994;
330:
1639-1644.
10.
Global
strategy
for
the
diagnosis,
management
and
prevention
of
chronic
obstructive
pulmonary
disease
2010.
Global
initiative
for
chronic
obstructive
lung
disease
(GOLD).
11.
Fisher
MR,
Forfia
PR,
Chamera
E,
Housten-Harris
T,
Champion
HC,
Girgis
RE,
et
al.
Accuracy
of
Doppler
echocardiography
in
the
hemodynamic
assessment
of
pulmonary
hypertension.
Am
J
Respir
Crit
Care
Med
2009;
179:
615-621.
12.
Schamroth
L.
Personal
experience.
S
Afr
Med
J
1976;
50:
297-300.
13.
Vandemergel
X,
Renneboog
B.
Prevalence,
aetiologies
and
significance
of
clubbing
in
a
department
of
general
internal
medicine.
Eur
J
Intern
Med
2008;
19:
325-329.
14.
Helvaci
MR,
Aydin
Y,
Ayyildiz
O.
Hydroxyurea
may
prolong
survival
of
sickle
cell
patients
by
decreasing
frequency
of
painful
crises.
HealthMED
2013;
7:
2327-2332.
15.
Mathers
CD,
Sadana
R,
Salomon
JA,
Murray
CJ,
Lopez
AD.
Healthy
life
expectancy
in
191
countries,
1999.
Lancet
2001;
357:
1685-1691.
16.
Helvaci
MR,
Ayyildiz
O,
Gundogdu
M.
Gender
differences
in
severity
of
sickle
cell
diseases
in
non-smokers.
Pak
J
Med
Sci
2013;
29:
1050-1054.
17.
Helvaci
MR,
Kaya
H.
Effect
of
sickle
cell
diseases
on
height
and
weight.
Pak
J
Med
Sci
2011;
27:
361-364.
18.
Helvaci
MR,
Kaya
H,
Duru
M,
Yalcin
A.
What
is
the
relationship
between
white
coat
hypertension
and
dyslipidemia?
Int
Heart
J
2008;
49:
87-93.
19.
Helvaci
MR,
Kaya
H,
Sevinc
A,
Camci
C.
Body
weight
and
white
coat
hypertension.
Pak
J
Med
Sci
2009;
25:
916-921.
20.
Parfrey
NA,
Moore
W,
Hutchins
GM.
Is
pain
crisis
a
cause
of
death
in
sickle
cell
disease?
Am
J
Clin
Pathol
1985;
84:
209-212.
21.
Helvaci
MR,
Sevinc
A,
Camci
C,
Keskin
A.
Atherosclerotic
background
of
cirrhosis
in
sickle
cell
patients.
Pren
Med
Argent
2014;
100:
127-133.
22.
Helvaci
MR,
Gokce
C.
Painful
crises
and
survival
of
sickle
cell
patients.
HealthMED
2014;
8:
598-602.
23.
Miller
ST,
Sleeper
LA,
Pegelow
CH,
Enos
LE,
Wang
WC,
Weiner
SJ,
et
al.
Prediction
of
adverse
outcomes
in
children
with
sickle
cell
disease.
N
Engl
J
Med
2000;
342:
83-89.
24.
Balkaran
B,
Char
G,
Morris
JS,
Thomas
PW,
Serjeant
BE,
Serjeant
GR.
Stroke
in
a
cohort
of
patients
with
homozygous
sickle
cell
disease.
J
Pediatr
1992;
120:
360-366.
25.
Cole
TB,
Sprinkle
RH,
Smith
SJ,
Buchanan
GR.
Intravenous
narcotic
therapy
for
children
with
severe
sickle
cell
pain
crisis.
Am
J
Dis
Child
1986;
140:
1255-1259.
26.
Miller
BA,
Platt
O,
Hope
S,
Dover
G,
Nathan
DG.
Influence
of
hydroxyurea
on
fetal
hemoglobin
production
in
vitro.
Blood
1987;
70:
1824-1829.
27.
Platt
OS.
Is
there
treatment
for
sickle
cell
anemia?
N
Engl
J
Med
1988;
319:
1479-1480.
28.
Helvaci
MR,
Aydogan
F,
Sevinc
A,
Camci
C,
Dilek
I.
Platelet
and
white
blood
cell
counts
in
severity
of
sickle
cell
diseases.
Pren
Med
Argent
2014;
100:
49-56.
29.
Charache
S.
Mechanism
of
action
of
hydroxyurea
in
the
management
of
sickle
cell
anemia
in
adults.
Semin
Hematol
1997;
34:
15-21.
30.
Brawley
OW,
Cornelius
LJ,
Edwards
LR,
Gamble
VN,
Green
BL,
Inturrisi
CE,
et
al.
NIH
consensus
development
statement
on
hydroxyurea
treatment
for
sickle
cell
disease.
NIH
Consens
State
Sci
Statements
2008;
25:
1-30.
31.
Tefferi
A.
Polycythemia
vera
and
essential
thrombocythemia:
2012
update
on
diagnosis,
risk
stratification,
and
management.
Am
J
Hematol
2012;
87:
285-293.
32.
Campion
SN,
Davenport
SJ,
Nowland
WS,
Cappon
GD,
Bowman
CJ,
Hurtt
ME.
Sensitive
windows
of
skeletal
development
in
rabbits
determined
by
hydroxyurea
exposure
at
different
times
throughout
gestation.
Birth
Defects
Res
B
Dev
Reprod
Toxicol
2012;
95:
238-249.
33.
Charache
S,
Barton
FB,
Moore
RD,
Terrin
ML,
Steinberg
MH,
Dover
GJ,
et
al.
Hydroxyurea
and
sickle
cell
anemia.
Clinical
utility
of
a
myelosuppressive
"switching"
agent.
The
Multicenter
Study
of
Hydroxyurea
in
Sickle
Cell
Anemia.
Medicine
(Baltimore)
1996;
75:
300-326.
34.
Steinberg
MH,
Barton
F,
Castro
O,
Pegelow
CH,
Ballas
SK,
Kutlar
A,
et
al.
Effect
of
hydroxyurea
on
mortality
and
morbidity
in
adult
sickle
cell
anemia:
risks
and
benefits
up
to
9
years
of
treatment.
JAMA
2003;
289:
1645-1651.
35.
Lebensburger
JD,
Miller
ST,
Howard
TH,
Casella
JF,
Brown
RC,
Lu
M,
et
al;
BABY
HUG
Investigators.
Influence
of
severity
of
anemia
on
clinical
findings
in
infants
with
sickle
cell
anemia:
analyses
from
the
BABY
HUG
study.
Pediatr
Blood
Cancer
2012;
59:
675-678.
|
|
.................................................................................................................

|
| |
|

