|
The effects of Matricaria
Chamomilla extract during neonatal period of
rats on pituitary-gonadal hormone axis and changes
in testicular tissue of male progenies
Safieh Golkhani (1,2)
Akbar Vahdati (2)
Mehrdad Modaresi (3)
Mohammad Amin Edalatmanesh (2)
(1) Department of Biology , College of Science,
Fars Science and Researches Branch, Islamic
Azad University, Fars, Iran
(2) Department of Biology, College of Science,
Shiraz Branch, Islamic Azad University, Shiraz,
Iran
(3) Animal Sciences Department, Isfahan (Khorasgan)
Branch, Islamic Azad University, Isfahan, Iran
Correspondence:
Akbar Vahdati
Department of Biology,
College of Science, Shiraz Branch,
Islamic Azad University,
Shiraz, Iran
|
Abstract
German
Chamomile (Matricaria Chamomilla) is from
the Astraceae family. This plant has been
used in traditional medicine such as analgesic,
antispasmodic and anti-inflammatory drugs
and for treating skin diseases and so
on. In this study, the effects of using
German chamomile’s hydroalcoholic
extract during the neonatal period were
evaluated on pituitary-gonadal hormone
axis and changes in testicular tissue
of male rat progenies. Forty female mature
virgin rats from the Wistar race, in the
weight range of 180-200g and age range
of 90-100 days, were used. After childbirth,
samples were divided into four groups
(ten mice per group) including control,
placebo, and two experimental groups.
Control group did not receive injections.
Placebo group was injected with 0.5cc
of normal saline daily during the lactation
period. Experimental groups received 50mg/kg
and 100mg/kg of hydroalcoholic extract
obtained by soaking method. Injections
were done intraperitoneally every day
during the lactation period. At the end
of the period (24 days), blood samples
were taken from heart, serum was separated
and the amount of testosterone, FSH, and
LH were measured. Also, testis tissue
slides were prepared and colored using
eosin-hematoxylin method and studied histologically.
Results showed that the extract increased
FSH not significantly whereas increased
LH, testosterone and also male sexual
cells including spermatogonia, spermatocytes,
spermatids, Sertoli cells, and Leydig
cells significantly (P<0.05).
Key words: Chamomile,
hydroalcoholic extract, neonatal, pituitary
gonadal axis, testis tissue, testosterone,
LH, FSH
|
The use of herbs is as old as human creation.
By studying the ancient tribes, we find that
medicinal plants have been used as medicine,
pesticides, detergents, paints and so on. Some
extant chemical compounds in plants have a complex
structure that it is impossible to synthesize
in the laboratory or is possible only by spending
a lot of time and money. After facing problems
such as water, air and soil pollution which
have been caused by chemical factors and also
side effects of chemical drugs which often appear
after a few generations, the use of nondestructive
matters was proposed so that herbal drugs were
used more than 7% in industrialized countries
(Zargari, 2008, Zaman 1989).
Due to adverse effects and side effects of
chemical drugs, using medicinal plants has been
considered of late. Many studies have been conducted
about the effects of various plants on fertility
of laboratory mammals which have presented valuable
results (Parandin et al. 2011).
Fertility is one of the most important issues
in medicine. The most common reason for men’s
infertility is their inability in producing
male sexual hormones and sufficient active healthy
sperms (Kumar and Kant Singh. 2015). Spermatogenesis
in the testis is carried out under the control
of secreted testosterone and secretion action
of testes is controlled itself by hypothalamic-pituitary-testicular
axis (Ramaswamy and Weinbauer. 2014).
Chamomile (Matricaria Chamomilla) from Astraea
family has been proposed in traditional medicine
because of its different properties. It is a
fragrant plant which grows in lawns and gravel
courts. Chamomile has a green white stem, and
small hairy leaves with narrow irregular cuts
(Esmaeili et al. 2007). The origin of this plant
is from different parts surrounding the Mediterranean
Sea but is now found in Europe, Moderate regions
of Asia, and even in America. Chamomile is used
in traditional medicine as a pain reliever and
anti-depression drug (Viapiana et al. 2016).
Chamomile is also used for treating many human
diseases such as hay fever, inflammation, muscle
spasms, menstrual disorders, insomnia, ulcers,
digestive disorders, rheumatic pain, and hemorrhoids
(Srivastava et al. 2010). Also, scientists have
reported positive effects of chamomile on clinical
and laboratory symptoms of polycystic ovaries
(Zafari Zangeneh et al. 2010).
Chamomile contains flavonoids such as apigenin
and luteolin, volatile oils such as bisabolol
chamazulene, and sesquiterpene, lactones including
matricarin, mucilage contains polysaccharides,
capric and nonilik ethers amino acids, fatty
acids, phenolic acids, and other compounds (Johari
et al. 2015). Previous studies have shown that
extant compounds in chamomile’s extract
have anti-bacterial, anti-inflammatory properties
and anti-oxidant activity. This plant is full
of flavonoids which have effective anti-oxidants
for neutralizing oxygen radicals (Hatami and
Estakhr. 2013).
Free oxygen species are capable of lipid peroxidation
in sperm membrane which is followed by reduced
mobility and damages to membrane parts of sperm.
Anti-oxidants are compounds which prevent formation
of free radicals and peroxidation of lipids,
protect sperm cells from free radicals and improve
sperm quality and fertility parameters (Maneesh
and Jayalskhmi. 2006). Medicinal plants have
positive effects on fertility increment, hormonal
imbalances, sexual dysfunction and have been
considered from ancient times.
Chamomile is dry and warm according to traditional
medicine of Iran, and has been used as a sexual
stimulant. Chemical studies on this plant have
shown large amounts of anti-oxidants (Hatami
and Estakhri. 2013).
Since the efficacy of herbal medicines must
be proven in clinical trials, and because few
have studied the effects of chamomile’s
extract on male reproductive activity and testicular
function, this study was carried out to investigate
the effects of German chamomile’s extract
during the neonatal period of rats on pituitary-gonadal
hormone axis and changes in testicular tissue
of male progenies.
The study was conducted in the animal nest
of Islamic Azad University- Falavarjan Branch
(2016). Forty virgin mature female rats from
Wistar race, in the weight range of 180-200g
and age range of 90-100 days were used as parents.
To adapt to the environment, samples were kept
under 22 to 26 ° C, 40-60% humidity and
natural photo period with free access to water.
Also, 10 adult male Wistar rats were used for
mating.
At first, 100 micrograms of estradiol valerate
was dissolved in olive oil and injected intramuscularly
to synchronize the ovulation time of rats. After
42 hours, 50 micrograms of progesterone was
injected intramuscularly (Hosseini et al. 2013).
Six hours later, vaginal smears were taken from
rats using swab moistened with saline. Immediately
after spreading the sample on a slide, 96% ethanol
was added to stabilize them and they were dried
in the air. Then, slides were colored using
Gimsa solution which was diluted at a ratio
of 1 to 20 (Jamil et al. 2013).
According to the proportions and morphology
of leukocytes and epithelial cells, estrus cycle
stages were determined. So that in proestrus
stage nucleated epithelial cells were dominant,
in estrous phase, horn cells without nuclei
and during the next stage Metestrus, the same
percentage of horn cells, epithelial cells and
leukocytes were observed. In diestrus stage
leucocytes were dominant (Hubscher et al. 2005,
Marcondes et al. 2002).
Microscopic observations showed that rats had
been synchronized at Estrous stage. Rats were
divided into four members’ groups with
a male rate for mating and kept for one night.
By observing vaginal plug day zero of pregnancy
was designated and then male rats were separated
and samples were divided into four groups (10
rats in each group) including control, placebo,
and two experimental groups. Control group received
no treatment. Placebo group received 0.5 cc of
normal saline for 24 days as injection stress.
Experimental groups received 50 and 100 mg/kg
weight of hydroalcoholic extract intraproteonal
every day during location.
Herbal samples were prepared from Isfahan Agricultural
Research Center and the extract was prepared
using soaking method.
Male and female progenies were separated from
day 24 which is the end of lactation and were
kept until maturity (two months). After that,
male progenies were anesthetized by intraperitoneal
injection of 0.7 mg/kg ketamine 10 % and blood
samples were taken from the heart. For separation
of serum, special test tubes were used. Samples
were centrifuged for 15 minutes (300 cycles/
minute).
Then, serum was separated from clot and the
amount of FSH and LH hormones were measured
using electrochemiluminescence (ECC- SIMENS)
whereas testosterone was measured using Elisa
method (state fax 2100). Also, testis tissues
were placed in formalin 10%. Then, some slides
were taken from every tissue sample and after
dehydration, clarification, parraffinization,
molding and preparation of tissue sections by
microtome stages, slides with 5 micrometer thickness
were prepared, colored by eosin-hematoxylin
method and studied using light microscopy.
Obtained data were analyzed using SPSS. One-way
analysis of variance and Tukey’s mean comparison
test were used at 5% probability level.
Pituitary-
gonadal
axis
hormones
Variance
analysis
results
showed
that
Follicle
stimulating
hormone
(FSH)
of
experimental
groups
(50
and
100
mg/kg
of
extract)
were
increased
in
proportion
to
control
and
placebo
groups
during
neonatal
period
but
not
significantly
(Table
1).
Luteinizing
hormone
(LH)
of
experimental
groups
(50
and
100
mg/kg
of
extract)
were
increased
in
proportion
to
control
and
placebo
groups
during
neonatal
period.
The
increment
was
not
significant
for
50
mg/kg
but
was
significant
for
100
mg/kg
(Table
1).
Testosterone
was
increased
significantly
(P<0.05)
by
experimental
groups
during
neonatal
period
(Table
1).
Table
1:
Comparison
the
mean
serum
level
of
LH,
FSH
hormones
and
testosterone
in
the
groups
treated
with
HEG
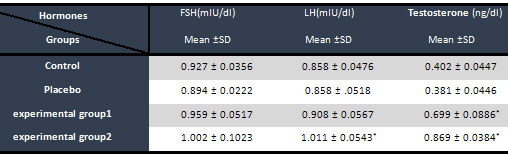 *Shows
significant
difference
from
control
group
(P<0.05) *Shows
significant
difference
from
control
group
(P<0.05)
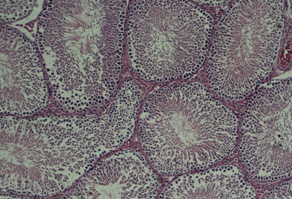
Testis
tissue
Microscopic
studies
of
testis
sides
did
not
show
significant
differences
between
various
groups.
In
these
samples,
tissue
appearance
was
normal
with
somniferous
tubules
which
had
spermatogenesis
cells
and
tubular
connective
tissue.
It
must
be
mentioned
that
better
appearance
of
experimental
groups
shows
stimulating
effect
of
chamomile
on
spermatogenesis.
Average
and
standard
deviation
of
spermatogonia
are
presented
in
Table
2.
Statistical
analysis
showed
significant
increases
in
experimental
groups
in
proportion
to
control
group.
Statistical
analysis
showed
significant
increases
in
the
number
of
spermatocytes
in
the
experimental
groups
in
proportion
to
control
group.
(Table
2)
Table
2
shows
significant
increase
in
spermatid
number
of
experimental
groups
in
proportion
to
control
group
during
neonatal
period
(P<0.05).
The
number
of
Sertoli
cells
was
increased
significantly
in
experimental
groups
(Table
2)
The
number
of
Leydig
cells
showed
significant
increases
in
experimental
groups
in
proportion
to
control
group.
(Table
2)
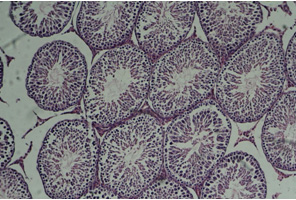
Figure
1:
Cross-section
of
testis,
control
group
(10X)
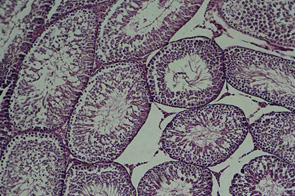
Figure
2:
Cross-section
of
testis,
placebo
group
(10X)
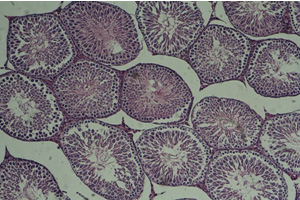
Figure
3:
Cross-section
of
testis,
first
experimental
group
(10X)
Figure
4:
Cross-section
of
testis,
second
experimental
group
(10X)
Click
here
for
Table
2:
the
number
of
lineage
sex
cells
in
the
groups
treated
with
HEG
in
comparison
to
control
Results
of
this
study
showed
that
hydro
alcoholic
extract
of
chamomile
did
not
change
testis
structure
of
rats.
However,
the
amount
of
spermatogonia,
spermatocytes,
spermatids,
Sertoli
and
Leydig
cells
were
significantly
increased
by
the
extract.
Also,
FSH
amount
was
increased
but
not
significantly
and
LH
was
significantly
increased
by
100
mg/kg
group.
Testosterone
was
increased
significantly
in
both
experimental
groups.
Also,
the
number
of
spermatogonia,
spermatocytes,
spermatids,
Sertoli
and
Leydig
cells
were
increased
in
experimental
groups
dose
dependently.
The
increment
may
be
because
of
extant
compounds
in
chamomile
which
affect
hypothalamic-pituitary-
testis
axis
and
increased
mentioned
hormones.
This
axis
itself
can
be
affected
by
various
controlling
factors
(negative
and
positive).
Previous
studies
have
shown
that
testosterone
plays
an
improvement
role
in
nourishing
the
dividing
sexual
cells
by
direct
effect
on
Sertoli
cells,
secretion
of
seminiferous
tubules
liquid
and
various
proteins
such
as
growth
factor
and
transferrin
(Carlson.
2012).
In
Hatami
and
Estakhr’s
study
(2013),
the
number
of
spermatogonia,
spermatocytes
and
spermatids
were
increased
by
chamomile
extract.
In
that
study,
FSH
amount
of
the
treatment
group
was
not
significantly
different
from
the
control
group
and
LH
and
testosterone
hormones
were
significantly
increased
by
the
extract
which
is
in
agreement
with
our
results.
Capuzzoa
et
al.
(2014)
reported
that
anti-oxidant
power
chamazulene
in
chamomile
was
much
more
than
ascorbic
acid
(vitamin
C).
Chamomile
is
rich
of
flavonoids
and
phenolic
compounds
which
are
effective
antioxidants
for
neutralizing
oxygen
free
radicals
(Pekka
et
al.
1996).
Antioxidants
are
probable
mechanisms
of
chamomile’s
effects
on
sperm
increment.
Crocetin
comes
from
phenolic
compounds
of
chamomile’s
extract
(Karbalaydoust.
2009).
This
matter
is
used
for
storing
sperm
under
very
low
temperatures
because
of
its
anti-oxidant
effects
(Henkel.
2005).
Also,
anti-oxidant
properties
of
phenolic
compounds
eliminate
free
radicals
and
affect
sperm
relating
factors
(Gill-Guzman
et
al.
2001).
Acharya
et
al.
(2008)
showed
that
reduced
activity
of
antioxidant
enzymes
decreased
the
number
of
sperm
but
following
administration
of
antioxidants
total
number
of
sperm
was
increased.
Chamomile
extract
contains
phytoestrogen
compounds
which
are
from
prolactin
secretion
stimulating
factors.
Prolactin
increment
causes
down-regulation
of
luteinizing
hormone
(LH)
in
Leydig
cells,
reduction
in
enzymes
involved
in
steroidogenesis
and
finally
testosterone
reduction
which
cholesterol
is
its
synthesis
prerequisite.
Also,
phytosterols
of
chamomile’s
extract
reduce
steroidal
hormones
such
as
testosterone
by
reducing
cholesterol
amount
(Wilson
and
Foster.
2003,
Hannana
et
al.
2003,
Shingo
et
al.
2015).
Johari
et
al.
(2015)
studied
the
effects
of
chamomile’s
extract
on
serum
concentrations
of
testosterone
and
gonadotropins
in
male
rats
and
reported
that
chamomile
reduced
the
amount
of
testosterone
but
didn’t
affect
gonadotropins
and
announced
that
phytoestrogen
existence
was
the
reason
for
testosterone
reduction.
These
results
are
opposed
to
our
results
which
can
be
due
to
dose
difference
or
consumption
time
of
extract
at
maturity
or
neonatal
periods.
Since
free
radicals
are
produced
in
daily
reactions
of
body
and
affect
reduction
of
sperm
number
and
its
mobility
(Gill-Gursman
et
al.
2001)
and
due
to
the
fact
that
laboratory
animals
experience
stress
because
of
living
in
closed
spaces,
chamomile
has
probably
had
positive
effects
on
spermatogenesis
because
of
its
anti-oxidants
including
chamazulene
(a
powerful
anti-oxidant).
Chamomile
with
its
anti-oxidant
properties
can
improve
the
process
of
making
sperm
plus
increase
in
sexual
hormones.
According
to
results,
existence
of
antioxidant
reduces
negative
effects
of
phytoestrogens
on
performance
of
pituitary-gonadal
axis
and
spermatogenesis
process
but
more
studies
are
needed.
Acharya,
UR.
Mishra,
M.
and
Patro,
J.
2008.
Effect
of
vitamins
C
and
E
on
spermatogenesis
in
mice
exposed
to
cadmium.
Reprod
Toxicol.
25(1):84-8.
Amsterdam,
J.
D.
Shults,
J.
Soeller,
I.
Mao,
J.
J.
Rockwell,
K.and
Newberg,
A.
B.
(2012).
Chamomile
(Matricaria
recutita)
may
have
antidepressant
activity
in
anxious
depressed
humans
–
an
exploratory
study.
Altern.
Ther.
Health
Med.
18,
44–49.
Capuzzoa,A.
Occhipintiab,
A
and.
Maffei,
M.E.
2014.
Antioxidant
and
radical
scavenging
activities
of
chamazulene.
Natural
Product
Research.
28(
24):
2321–2323.
Carlson,
B.M..
2012.
Human
embryology
and
developmental
biology.
5th
ed.
University
of
Michigan,
Michigan,
Elsevier,
506p.
Esmaeili,
M.
Honarvaran,
F.
Kesmati,
M.
Jahani
Hashemi,
H.and
Jafari,
H.
Effects
of
matricaria
chamomilla
extract
on
Morphine
withdrawal
syndrome
in
mice.
The
Journal
of
Qazvin
University
of
Medical
Sciences.
43(2):13-8.
[in
Persian]
Gill-Guzman,
E.
Ollero,
M.
Lopez,
M.C.
Sharma,
R.K.
and
Alavarez,
J.G.
2001.
Differential
production
of
reactive
oxygen
species
by
subsets
of
human
spermatozoa
at
different
stages
of
maturation.
Hum
Reprod,
16(9):1922-30.
Hannana,
J.M.A.
Rokeyaa,
B.
Faruquea,
O.
Naharb,
N.
Mosihuzzamanb,
M.
Azad
Khana,
A.K.
and
Ali,
L.
2003.
Effect
of
soluble
dietary
fibre
fraction
of
Trigonella
foenum
graecum
on
glycemic,
insulinemic,
lipidemic
and
platelet
aggregation
status
of
Type
2
diabetic
model
rats.
Journal
of
Ethnopharmacology.
88(1):73-77.
Hatami,
L.
and
Estakhr,
J.
2013.
The
Effects
of
Hydroalcoholic
Extract
of
Matricaria
Recutita
on
the
Hormonal
Pituitary-Testis
Axis
and
Testis
Tissue
Changes
of
Mature
Male
Rats
.
Journal
of
Fasa
University
of
Medical
Sciences.
3(1):56-62.
Hormonal
and
testicular
tissue
in
mice.
Journal
of
Animal
Environment.
8(1):51-56.[
in
Persian]
Henkel,
R.
2005.
The
impact
of
oxidants
on
sperm
functions.
Journal
Compilation.
37(6):205-206.
Hosseini,
E
.
Frozanfar,
M
.
and
Payehdar,
A.
2013.
The
effect
of
hydroalcoholic
extract
of
purslane
on
serum
concentration
of
esterogen,
progesterone,
prolactin
and
gonadotropins
in
mature
female
rats.
Journal
of
Shahrekord
University
of
Medical
Sciences,
15(5):
1221.
[in
Persian]
Hubscher,
CH.
Brooks,
DL.
and
Johnson,
JRA.
2005.
Quantitative
method
for
assessing
stages
of
the
rat
estrous
cycle.
Biotechnic
&
Histochemistry,
80(2):
79-87.
Jamil,
F.
Behnam
Rassouli,
M.
Mahdavi
Shahri,
N.
and
Dehghani,
H.
2013.
Study
of
the
Effects
of
Hyperglycemia
and
Insulin
Therapy
on
Uterus
Histology
and
Estrous
cycle
in
Wistar
Rat.
Journal
of
Cell
&
Tissue,
4(2):
149-157.
[in
Persian]
Johari,
H.
Khavarian,
M.
Moghtari,
M.
Kamali,
M.
and
Kargar
Jahromi,
H.
2014.
Effects
of
hydroalcoholic
extract
of
matricaria
chamomilla
flower
on
testosterone
and
gonadotropins
in
adult
male
rats.
Pars
Journal
of
Medical
Sciences.
12(4):31-36.
Karbalay-doust,
S.
2009.
Antiulcerogenic
effects
of
Matricaria
chamomilla
extract
in
experimental
gastric
ulcer
in
mice.
Iranian
Journal
of
Medical
Sciences.
34(3):198-203.
Kumar,N
and
Kant
Singh,A.
2015.
Trends
of
male
factor
infertility,
an
important
cause
of
infertility:
A
review
of
literature.
Journal
of
Human
Reproductive
Sciences.
8(4):
191–196.
Maneesh,
M
.
and
Jayalekshmi,
H.
2006.
Role
of
reactive
oxygen
species
and
antioxidants
on
pathophysiology
of
male
reproduction.
Indian
Journal
of
Clinical
Biochemistry.
21(2):80-89.
Marcondes,
FK.
Bianchi,
FJ.
and
Tanno,
AP.
2002.
Determination
of
the
estrous
cycle
phase
of
the
rats:
some
helpful
considerations.
Brazilian
Journal
of
Biology,
62:
609-614.
Parandin,
R
.
Ghorbani,R.
and
Sadeghipour
Roodsari
,HR.
2011.
Effects
of
Alcoholic
Extract
of
Achillea
Millefolium
Flowers
on
Fertility
Parameters
in
Male
Rats.
Journal
of
Shahid
Sadoughi
University
of
Medical
Sciences
.
19(1):84-93.
Ramaswamy
,
S.
and
Weinbauer
,
G.F.
2014.
Endocrine
control
of
spermatogenesis:
Role
of
FSH
and
LH/
testosterone
.
Spermatogenesis
.
4(2).
Rekka,
EA.
Kourounakis,
AP.and
Kourounakis,
PN.
1996.
Investigation
of
the
effect
of
chamazulene
on
lipid
peroxidation
and
free
radical
processes.
Res
Commun
in
Mol
Pathol
&
Pharamacol.
92(3):361
-
364.
Shingo,
T.
Gregg,Ch.
Enwere,
E.
Fujikawa,
H.
and
Hassam,
R.
2003.
Pregnancy-Stimulated
Neurogenesis
in
the
Adult
Female
Forebrain
Mediated
by
Prolactin.
Science.
299:117-120.
Srivastava,
J.K.
.
Shankar,
E
.
and
Gupta,
S.
2010.
Chamomile:
A
herbal
medicine
of
the
past
with
bright
future.
Author
manuscript.
3(6):895-901.
Viapiana,A.
Struck-Lewicka,W.
Konieczynski,P.
Wesolowski,M.
and
Kaliszan,
R.
2016.
An
Approach
Based
on
HPLC-Fingerprint
and
Chemometrics
to
Quality
Consistency
Evaluation
of
Matricaria
chamomilla
L.
Commercial
Samples.
Frontiers
in
plant
Sciences
.
7,1561
Article
.
Wilson
JD,
Foster
DW.
2015.
Textbook
of
Endocrinology,
13th
ed.
Elsevier.1936p.
Zafari
Zangeneh,F.
Minaee,
B.
Amirzargar,
A.
Ahangarpour,
A.
and
Mousavizadeh,
K.
2010.
Effects
of
Chamomile
Extract
on
Biochemical
and
Clinical
Parameters
in
a
Rat
Model
of
Polycystic
Ovary
Syndrome.
Journal
of
Reproduction
&
Infertility.
11(3):169-174.
Zaman,
S.2008
.Medicinal
Plants(Jiri
Stodola
and
Jan
Volak).
7th
ed.Ghoghnos
,
370p.
Zargari,
A.
1989.
Medicinal
Plants.
Volume
III.
Tehran
University,
923p.
.[
in
Persian]
|

