Of
all
the
patients,
three
patients
in
group
A
and
one
patient
in
group
B
were
excluded
from
the
study;
as
a
result,
the
remaining
96
patients
were
randomly
assigned
to
the
two
groups
receiving
sequential
therapy
regimen
with
levofloxacin
(49
patients)
and
triple
therapy
regimen
(47
patients).
The
mean
age
of
the
patients
in
the
sequential
therapy
group
and
triple
therapy
group
was
33.29
±
1.54
years
and
45.53
±
2.17
years,
respectively.
There
was
a
significant
difference
between
the
two
treatment
groups
in
terms
of
the
mean
age
(p
=
0.00).
However,
there
was
no
significant
difference
between
the
two
treatment
groups
in
terms
of
patients’
sex
distribution
(p
=
0.57).
Moreover,
there
was
also
a
significant
difference
between
the
two
treatment
groups
in
terms
of
the
distribution
of
patients
in
different
education
groups
(p
=
0.00).
Table
1
presents
the
data
on
patients’
age,
sex,
and
education
level.
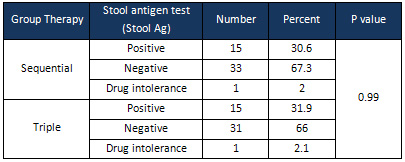
Of
the
patients
in
the
two
groups,
67.3%
of
the
patients
in
the
sequential
therapy
group
and
66%
of
the
patients
in
the
triple
therapy
group
had
negative
HpSA
(H.
pylori
Stool
Antigen);
there
was
no
significant
difference
between
the
two
treatment
groups
in
terms
of
the
eradication
of
H.
pylori
(p
=
0.99)
(Table
2).
In
addition,
concerning
the
side
effects
of
the
medications,
one
person
(2%)
in
the
sequential
therapy
group
(because
of
the
nausea
caused
by
amoxicillin)
and
one
person
(2.1%)
in
the
triple
therapy
group
(because
of
the
cramps
caused
by
clarithromycin)
were
unable
to
tolerate
the
drug.
However,
the
rest
of
the
participants
in
this
study
did
not
report
any
treatment-specific
complaints.
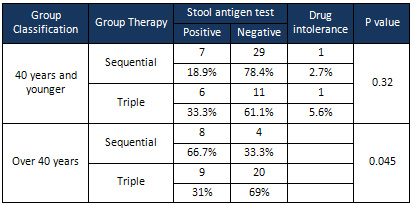
The
results
of
stool
antigen
test
were
used
to
assess
the
effects
of
patients’
age
on
the
efficacy
of
sequential
therapy
and
triple
therapy
for
the
eradication
of
H.
pylori.
The
results
showed
that
when
comparing
the
patients
aged
40
years
and
younger
between
the
two
treatment
groups,
there
was
no
significant
difference
between
them
in
terms
of
the
treatment
outcome
(p
=
0.32);
however,
when
comparing
patients
aged
over
40
years,
there
was
a
significant
difference
between
the
two
treatment
groups
in
terms
of
response
to
treatment.
Accordingly,
the
response
to
treatment
was
better
in
the
triple
therapy
group
(p
=
0.045)
(Table
3).
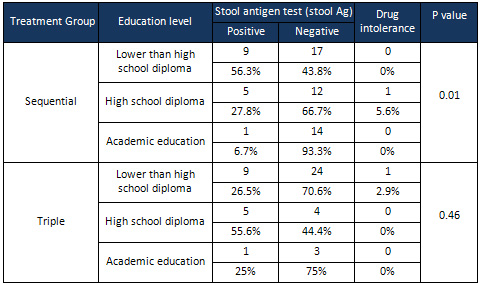
The
effects
of
education
levels
on
eradication
of
H.
pylori
were
assessed;
according
to
the
results,
the
responses
to
the
treatment
in
sequential
therapy
group
were
significantly
different
between
different
education
groups
(p
=
0.01),
but
in
the
triple
therapy
group
there
was
no
significant
difference
between
different
education
groups
in
terms
of
the
response
to
treatment
(p
=
0.46)
(Table
4).
There
was
a
significant
difference
between
the
two
groups
of
patients
with
education
levels
lower
than
high-school
diploma
and
academic
education
in
terms
of
response
to
treatment
(p
=
0.048).
There
was
also
a
slightly
significant
difference
between
the
two
groups
of
patients
with
high-school
diploma
and
academic
education
in
terms
of
response
to
treatment
(p
=
0.063).
However,
there
was
no
significant
difference
between
the
two
groups
of
patients
with
an
education
level
lower
than
high
school
diploma
and
with
high
school
diploma
in
terms
of
response
to
treatment
(p
=
0.89).
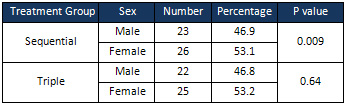
The
effect
of
sex
on
eradication
of
H.
pylori
was
also
assessed.
In
the
triple
therapy
group,
there
was
no
significant
difference
between
females
and
males
in
terms
of
response
to
treatment
(p
=
0.64);
however,
in
the
sequential
therapy
group,
a
significant
difference
was
observed
between
females
and
males
in
terms
of
response
to
treatment
(p
=
0.009).
Accordingly,
the
response
to
treatment
was
better
in
males
than
females
in
the
sequential
therapy
group
(Table
5).
Click
here
for
Table
1:
Demographic
data
of
patients
in
the
two
treatment
groups
receiving
triple
therapy
regimen
and
sequential
therapy
regimen
to
eradicate
H.
pylori
Table
2:
Comparison
of
the
results
of
stool
antigen
test
between
the
two
treatment
groups
receiving
triple
therapy
regimen
and
sequential
therapy
regimen
to
eradicate
H.
pylori
*
p
<
0.05
is
considered
as
significant
Table
3:
Comparison
of
the
effects
of
patients’
age
on
the
efficacy
of
treatment
(based
on
the
results
of
stool
antigen
test)
between
the
two
groups
receiving
triple
therapy
regimen
and
sequential
therapy
regimen
to
eradicate
H.
pylori
*
p
<
0.05
is
considered
as
significant
Table
4:
Comparison
of
the
effects
of
patients’
education
on
the
efficacy
of
treatment
(based
on
the
results
of
stool
antigen
test)
between
the
two
groups
receiving
triple
therapy
regimen
and
sequential
therapy
regimen
to
eradicate
H.
pylori
 *
p
<
0.05
is
considered
as
significant
*
p
<
0.05
is
considered
as
significant
Table
5:
Comparison
of
the
effects
of
patients’
sex
on
the
efficacy
of
treatment
(based
on
the
results
of
stool
antigen
test)
between
the
two
groups
receiving
triple
therapy
regimen
and
sequential
therapy
regimen
to
eradicate
H.
pylori
This
study
was
conducted
to
compare
standard
triple
therapy
regimen
with
sequential
therapy
regimen
containing
levofloxacin
used
for
the
eradication
of
H.
pylori
in
patients
with
gastrointestinal
infection
caused
by
H.
pylori.
H.
pylori
eradication
rate
was
67.3%
in
the
sequential
therapy
regimen
and
66%
in
standard
triple
therapy
regimen.
As
the
main
goal
of
treatment
is
to
eradicate
the
infection
in
85-95%
of
the
patients;
however,
because
of
the
lower
treatment
success
rates
observed
in
this
research,
hence,
these
two
regimens
in
this
study
is
not
recommended.
Several
studies
have
been
conducted
on
standard
triple
therapy
in
Iran.
In
a
study
by
Aminian
and
colleagues,
the
regimen
consisted
of
omeprazole
20
mg
twice
a
day,
amoxicillin
1
g
twice
a
day,
and
clarithromycin
500
mg
twice
a
day
which
had
been
administered
for
10
days
with
the
eradication
rate
of
90.7%
[30].
Moreover,
Keshavarz
and
colleagues
used
the
above-mentioned
treatment
regimen
for
seven
days
and
reported
an
eradication
rate
of
about
87.5%
[31].
Furthermore,
one
of
the
most
common
causes
of
treatment
failures
could
be
the
emergence
of
new
antibiotic-resistant
bacterial
strains
[32].
In
this
study,
the
emergence
of
H.
pylori
strains
resistant
to
the
antibiotics
might
have
been
a
cause
of
treatment
failure.
In
a
similar
study
conducted
by
Polat
and
colleagues,
of
a
total
of
72
patients
receiving
sequential
regimen
containing
levofloxacin,
65
patients
were
affected
by
gram-negative
bacteria,
while
of
a
total
of
67
patients
receiving
standard
triple
therapy
regimen,
only
34
patients
were
affected
by
gram-negative
bacteria
[29],
which
may
refer
to
the
fact
that
in
different
geographic
areas,
bacterial
resistance
to
antibiotics
might
be
different.
Because
of
the
common
complication
of
furazolidone
and
high
cost
of
clarithromycin,
many
Iranian
physicians
routinely
prefer
to
use
metronidazole,
amoxicillin,
bismuth,
and
omeprazole
for
H.
pylori
eradication
[4].
In
this
study,
the
patients
in
triple
therapy
for
ten
days
received
omeprazole
20
mg
twice
a
day,
amoxicillin
1
g
twice
a
day,
and
clarithromycin
500
mg
twice
a
day;
however,
the
patients
in
the
sequential
therapy
group
for
five
days
received
omeprazole
20
mg
twice
a
day
and
amoxicillin
1
g
twice
a
day;
in
the
next
five
days,
they
received
levofloxacin
250
mg
twice
a
day,
omeprazole
20
mg
twice
a
day,
and
tinidazole
500
mg
twice
a
day.
In
fact,
H.
pylori
resistance
to
these
antibiotics
might
have
been
a
reason
for
reduced
efficacy
of
the
regimens
in
eradication
of
the
bacterial
infection
in
this
research.
Long-term
clarithromycin
monotherapy
for
the
treatment
of
respiratory
tract
diseases
could
indeed
lead
to
the
increased
resistance
to
this
antibiotic.
H.
pylori
resistance
to
clarithromycin
might
also
be
the
most
important
factor
explaining
the
failure
of
treatment
regimens,
particularly
triple
therapy,
used
for
the
eradication
of
infection
[33-36].
Moreover,
it
is
reported
that
the
optimal
efficacy
of
metronidazole-based
triple
and
quadruple
drug
regimens
in
western
countries
is
about
80-95%
[5,
15].
However,
due
to
the
high
rates
of
resistance
to
metronidazole
in
Iran
[37,38],
the
eradication
rate
is
usually
not
optimal
[4,
39].
In
this
study,
the
use
of
the
mentioned
treatment
regimens
may
have
resulted
in
low
rates
of
H.
pylori
eradication
which
is
consistent
with
the
findings
of
Zhou
and
colleagues’
study
(in
2014)
who
reported
the
H.
pylori
resistance
to
sequential
and
triple
therapy
regimens
[40].
Studies
which
have
compared
sequential
and
triple
therapy
regimens
have
reported
different
results,
as
some
of
them
reported
the
superiority
of
sequential
treatment
regimen
while
other
others
have
reported
the
superiority
of
triple
therapy
regimen
[32,
41-44].
In
a
study
conducted
by
Khaleghi
and
colleagues,
the
patients
with
chronic
dyspepsia
were
classified
into
two
groups
each
consisting
of
80
people
[45].
One
of
the
groups
received
omeprazole
and
amoxicillin
for
the
first
five
days
and
then
omeprazole,
furazolidone,
and
clarithromycin
for
the
next
nine
days;
the
other
group
received
quadruple
regimen
for
14
days
consisting
of
omeprazole,
amoxicillin,
clarithromycin,
and
bismuth.
Of
all,
50.9%
of
the
patients
in
the
sequential
therapy
group
and
49.1%
of
the
patients
in
the
second
group
were
cured,
and
the
difference
was
not
statistically
significant
[45].
In
another
study,
Kaboli
and
colleagues
investigated
140
patients
with
dyspepsia
and
classified
them
into
two
groups;
the
first
group
received
omeprazole,
amoxicillin,
and
clarithromycin
for
14
days
and
the
second
group
(sequential
group)
first
received
omeprazole
and
amoxicillin
for
five
days,
and
then
omeprazole,
clarithromycin,
Tinidazole
for
the
next
five
days;
there
was
no
significant
difference
between
the
two
groups
in
terms
of
H.
Pylori
eradication
[46].
Zullo
and
colleagues
studied
36
patients
who
received
rabeprazole
20
mg
twice
a
day,
levofloxacin
250
mg
twice
a
day,
and
amoxicillin
1
g
twice
a
day.
According
to
the
results,
H.
pylori
was
successfully
treated
in
30
patients
[47].
In
fact,
comparing
with
the
sequential
regimen
used
in
this
study
and
the
obtained
results,
it
can
be
concluded
that
the
H.
pylori
strains
resistant
to
levofloxacin
might
have
been
caused
by
the
indiscriminate
use
of
antibiotics
in
this
region.
In
the
present
study,
considering
people
in
the
age
group
over
40
years,
the
eradication
rate
in
the
triple
therapy
group
(69%)
was
higher
than
the
eradication
rate
in
the
sequential
therapy
group
(33.3%)
(p
=
0.045).
To
justify
these
results,
it
can
be
concluded
that
the
use
of
sequential
therapy
regime,
especially
for
older
people,
is
more
difficult
than
that
of
triple
therapy
regimen.
The
more
complex
schedule
for
taking
sequential
therapy
regimen,
especially
in
people
over
40
years
of
age,
may
increase
the
risk
of
treatment
failure
in
this
study.
Nevertheless,
Hashemi
and
colleagues
(in
2007)
reported
that
patients’
age
had
no
significant
relationship
with
the
eradication
of
H.
pylori
[4].
Higher
education
level
is
reported
as
a
factor
influencing
the
eradication
of
H.
pylori
infection.
In
this
study,
the
highest
level
of
eradication
was
observed
in
people
with
an
academic
degree.
These
findings
are
in
line
with
other
studies
in
this
field
that
have
proven
H.
pylori
infection
is
lower
among
people
with
higher
education
levels
[48-50].
People
with
lower
education
level
are
indeed
at
a
higher
risk
of
infection
than
those
with
higher
education
levels;
such
a
negative
relationship
is
also
observed
between
parents’
education
and
infection
[51,52].
The
results
of
this
study
showed
that
men
had
a
better
response
to
treatments
than
women.
It
is
inconsistent
with
the
results
of
studies
by
Misattari
and
Hashemi
which
reported
no
statistically
significant
difference
between
men
and
women
in
terms
of
the
response
to
treatments;
however,
in
this
study
in
patients
in
the
triple
therapy
group,
there
was
no
significant
difference
between
the
two
sexes
in
terms
of
response
to
treatments
[4,
32,
53].
Given
the
low
rate
of
H.
Pylori
eradication
by
the
sequential
and
triple
therapy
regimens
observed
in
this
study,
further
research
must
be
conducted
to
study
the
resistance
of
the
bacteria
to
the
studied
treatment
lines
and
antibiotics
in
Iran.
Considering
the
results
of
this
study,
it
is
also
recommended
to
utilize
other
treatment
regimens
to
achieve
higher
rates
of
eradication.
It
is
also
suggested
to
use
more
effective
and
simple
treatment
regimens
for
older
people
and
those
with
lower
education
levels.
The
use
of
sequential
therapy
regimen
containing
levofloxacin
for
the
eradication
of
H.
pylori
results
in
outcomes
which
are
less
than
the
optimal
levels.
However,
further
studies
in
this
field
are
needed
to
be
carried
out
with
larger
sample
size
in
different
places.
Acknowledgments
This
study
was
extracted
from
M.D
thesis
of
Kian
Kaveh-Zadeh
(Thesis
#1214).
[1]
Tamadon
MR,
Saberi
Far
M,
Soleimani
A,
Ghorbani
R,
Semnani
V,
Malek
F,
Malek
M.
Evaluation
of
noninvasive
tests
for
diagnosis
of
Helicobacter
pylori
infection
in
hemodialysis
patients.
J
Nephropathol.
2013
Oct;2(4):249-53.
[2]
Tamadon
MR.
The
treatment
of
Helicobacter
pylori
in
chronic
kidney
disease
patients.
Front
Biomed.
2016;1(1):e03.
[3]
Ardalan
MR,
Mardani
S,
Asgari-Savadjani
S,
Tamadon
MR,
Naghdifar
S,
Nasri
H.
An
update
on
Helicobacter
pylori
infection
in
renal
failure
patients.
Immunopathol
Persa.
2016;2(2):e10.
[4]
Hashemi
S,
Hagh
Azali
M,
Mirzaii
M,
Sohrabpour
A,
Mohammadnejad
M.
The
efficacy
of
two-week
metronidazole,
amoxicillin-based
quadruple
therapy
for
eradication
of
H.
pylori
infection
in
Iranian
patients.
Razi
journal
of
medical
Sciences
2007;
14:
203-8.
[5]
Van
der
Hulst
R,
Tytgat
G.
H.
pylori
and
peptic
ulcer
disease.
Scandinavian
Journal
of
Gastroenterology
1996;
31:
10-8.
[6]
Wroblewski
LE,
Peek
RM,
Wilson
KT.
H.
pylori
and
gastric
cancer:
factors
that
modulate
disease
risk.
Clinical
microbiology
reviews
2010;
23:
713-39.
[7]
Kafeshani
M.
Diet
and
immune
system.
Immunopathol
Persa.
2015;1(1):e04.
[8]
Baradaran
A,
Kafeshani
M,
Assari
S.
From
intestine
to
kidney;
a
narrative
literature
review.
Acta
Persica
Pathophysiol.
2016;1(1):e03.
[9]
Hajibabaei
K.
The
role
of
antioxidants
and
pro-oxidants
in
the
prevention
and
treatment
of
cancers.
Ann
Res
Antioxid.
2016;1(1):e09.
[10]
Laeeq
SM,
Majid
Z,
Mandhwani
R,
Luck
NH,
Mubarak
M.
Cytomegalovirus
induced
pseudotumor
of
the
colon
in
a
renal
transplanted
patient.
J
Nephropharmacol.
2017;6(2):62-64.
[11]
Amiri
M.
They
injury
induced
by
Helicobacter
pylori
infection
on
gastric
mucosa.
J
Inj
Inflamm.
2017;1(2):e01.
[12]
Malani
PN.
Harrison’s
principles
of
internal
medicine.
JAMA
2012;
308:
1813-4.
[13]
Persson
C,
Jia
Y,
Pettersson
H,
Dillner
J,
Nyrén
O,
Ye
WH.
pylori
seropositivity
before
age
40
and
subsequent
risk
of
stomach
cancer:
a
glimpse
of
the
true
relationship?
PloS
one
2011;
6:
e17404.
[14]
Eck
M,
Schmausser
B,
Haas
R,
Greiner
A,
Czub
S,
Muller-Hermelink
H.
MALT-type
lymphoma
of
the
stomach
is
associated
with
H.
pylori
strains
expressing
the
CagA
protein.
Gastroenterology
1997;
112:
1482-6.
[15]
Walsh
JH,
Peterson
WL.
The
treatment
of
H.
pylori
infection
in
the
management
of
peptic
ulcer
disease.
New
England
Journal
of
Medicine
1995;
333:
984-91.
[16]
Vilaichone
RK,
Mahachai
V,
Graham
DY.
H.
pylori
diagnosis
and
management.
Gastroenterology
clinics
of
north
america
2006;
35:
229-47.
[17]
Zojaji
H,
Talaie
R,
Mirsattari
D,
Haghazali
M,
Molaei
M,
Mohsenian
N,
Derakhshan
F,
Zali
M.
The
efficacy
of
H.
pylori
eradication
regimen
with
and
without
vitamin
C
supplementation.
Digestive
and
Liver
Disease
2009;
41:
644-7.
[18]
Malfertheiner
P,
Megraud
F,
O’Morain
C,
Bazzoli
F,
El-Omar
E,
Graham
D,
Hunt
R,
Rokkas
T,
Vakil
N,
Kuipers
EJ.
Current
concepts
in
the
management
of
H.
pylori
infection:
the
Maastricht
III
Consensus
Report.
Gut
2007;
56:
772-81.
[19]
Malekzadeh
R,
Mohamadnejad
M,
Siavoshi
F,
Massarrat
S.
Treatment
of
H.
pylori
infection
in
Iran:
low
efficacy
of
recommended
western
regimens.
Arch
Iranian
Med
2004;
7:
1-8.
[20]
Abadi
ATB,
Taghvaei
T,
Mobarez
AM,
Carpenter
BM,
Merrell
DS.
Frequency
of
antibiotic
resistance
in
H.
pylori
strains
isolated
from
the
northern
population
of
Iran.
The
Journal
of
Microbiology
2011;
49:
987-93.
[21]
Mohammadi
M,
Doroud
D,
Mohajerani
N,
Massarrat
S.
H.
pylori
antibiotic
resistance
in
Iran.
World
journal
of
gastroenterology
2005;
11:
6009.
[22]
Chisholm
S,
Teare
E,
Davies
K,
Owen
R.
Surveillance
of
primary
antibiotic
resistance
of
H.
pylori
at
centres
in
England
and
Wales
over
a
six-year
period
(2000-2005).
Euro
surveillance:
bulletin
Europeen
sur
les
maladies
transmissibles
=
European
communicable
disease
bulletin
2007;
12:
E3-4.
[23]
Boyanova
L,
Gergova
G,
Nikolov
R,
Davidkov
L,
Kamburov
V,
Jelev
C,
Mitov
I.
Prevalence
and
evolution
of
H.
pylori
resistance
to
6
antibacterial
agents
over
12
years
and
correlation
between
susceptibility
testing
methods.
Diagnostic
microbiology
and
infectious
disease
2008;
60:
409-15.
[24]
Bang
SY,
Han
DS,
Eun
CS,
Kim
JE,
Ahn
SB,
Sohn
JH,
Jeon
YC,
Kang
JO.
[Changing
patterns
of
antibiotic
resistance
of
H.
pylori
in
patients
with
peptic
ulcer
disease].
The
Korean
journal
of
gastroenterology
=
Taehan
Sohwagi
Hakhoe
chi
2007;
50:
356-62.
[25]
Hedaiaty
M,
Amiri
A,
Amiri
A.
Impact
of
proton
pump
inhibitors
on
renal
function
and
structure;
new
concepts.
J
Prev
Epidemiol.
2017;2(2):e05.
[26]
Hedaiaty
M,
Tamadon
MR,
Amiri
A,
Mahmoodnia
L.
Proton-pump
inhibitors
and
risk
of
renal
disease.
J
Nephropharmacol.
2017;6(2):33-37.
[27]
Amiri
M.
Renal
injury
by
administration
of
proton
pump
inhibitors.
J
Renal
Endocrinol.
2017;
3(2):e06.
[28]
Vaira
D,
Zullo
A,
Vakil
N,
Gatta
L,
Ricci
C,
Perna
F,
Hassan
C,
Bernabucci
V,
Tampieri
A,
Morini
S.
Sequential
therapy
versus
standard
triple-drug
therapy
for
H.
pylori
eradication:
a
randomized
trial.
Annals
of
Internal
Medicine
2007;
146:
556-63.
[29]
Polat
Z,
Kadayifci
A,
Kantarcioglu
M,
Ozcan
A,
Emer
O,
Uygun
A.
Comparison
of
levofloxacin-containing
sequential
and
standard
triple
therapies
for
the
eradication
of
H.
pylori.
European
journal
of
internal
medicine
2012;
23:
165-8.
[30]
Aminian
K,
Ghanbari
A,
Joukar
F,
Farsad
F,
Shahrokhi
RR,
Mansour
GF.
Comparison
Of
Triple,
Quadruple
And
Two
Sequential
Drug
Therapy
For
Eradication
Of
H.
Pylori
Infection.
2010.
[31]
Keshavarz
A,
Izadi
B,
Rezaei
M,
Shahkarami
A.
A
comparative
study
of
eradication
of
H.
pylori
infection
in
dyspeptic
patients
using
a
low
dose
and
a
high
dose
triple
therapy
with
clarithromycin,
amoxicillin
and
Omeprazole.
Behbood
J
2009;
13:
20-7.
[32]
Mirsattari
D,
ShamsiAfzali
E,
Zojaji
H,
Naderi
N,
Almasi
S,
Khalilimaryan
E,
Sanati
A,
Zali
M.
New
Sequential
Versus
Triple
Treatment
Schedules
for
H.
pylori
Eradication
in
Iran.
Govaresh
2012;
17:
116-21.
[33]
Malfertheiner
P,
Megraud
F,
O’morain
C,
Hungin
A,
Jones
R,
Axon
A,
Graham
D,
H.
pylori
Tytgat
G.
Current
concepts
in
the
management
of
infection-The
Maastricht
2
-
in
2000
Consensus
Report.
Alimentary
pharmacology
&
therapeutics
2002;
16:
167-80.
[34]
Megraud
F.
H.
pylori
antibiotic
resistance:
prevalence,
importance,
and
advances
in
testing.
Gut
2004;
53:
1374-84.
[35]
Graham
DY,
Lu
H,
Yamaoka
Y.
A
report
card
to
grade
H.
pylori
therapy.
Helicobacter
2007;
12:
275-8.
[36]
Vakil
N,
Megraud
F.
Eradication
therapy
for
H.
pylori.
Gastroenterology
2007;
133:
985-1001.
[37]
Malekzadeh
R,
Setodeh
R,
Amini
M,
Vakili
A,
Ansari
R,
Massarat
S,
editors.
Effect
of
furazolidone,
bismuth
subcitrate
and
amoxicillin
on
eradication
of
H.
pylori
(Hp)
in
Iran.
Gastroenterology;
1997:
WB
SAUNDERS
CO-ELSEVIER
INC
1600
JOHN
F
KENNEDY
BOULEVARD,
STE
1800,
PHILADELPHIA,
PA
19103-2899
USA.
[38]
Siavashi
F,
Heydarian
E,
Pourkhajeh
A,
Merat
S,
ASLSOLEYMANI
H,
Khatibian
M,
Malekzadeh
R.
Susceptibility
of
various
strains
of
H.
pylori
to
selected
agents.
2000.
[39]
Malekzadeh
R,
Ansari
R,
Vahedi
H,
Siavoshi
F,
Alizadeh
B,
Eshraghian
M,
Vakili
A,
Saghari
M,
Massarrat
S.
Furazolidone
versus
metronidazole
in
quadruple
therapy
for
eradication
of
H.
pylori
in
duodenal
ulcer
disease.
Alimentary
Pharmacology
and
Therapeutics
2000;
14:
299-304.
[40]
Zhou
L,
Zhang
J,
Chen
M,
Hou
X,
Li
Z,
Song
Z,
He
L,
Lin
S.
A
comparative
study
of
sequential
therapy
and
standard
triple
therapy
for
H.
pylori
infection:
a
randomized
multicenter
trial.
The
American
journal
of
gastroenterology
2014;
109:
535.
[41]
Jafri
NS,
Hornung
CA,
Howden
CW.
Meta-analysis:
sequential
therapy
appears
superior
to
standard
therapy
for
H.
pylori
infection
in
patients
naive
to
treatment.
Annals
of
Internal
Medicine
2008;
148:
923-31.
[42]
Liou
JM,
Chen
CC,
Chen
MJ,
Chen
CC,
Chang
CY,
Fang
YJ,
Lee
JY,
Hsu
SJ,
Luo
JC,
Chang
WH.
Sequential
versus
triple
therapy
for
the
first-line
treatment
of
H.
pylori:
a
multicentre,
open-label,
randomised
trial.
The
Lancet
2013;
381:
205-13.
[43]
Kutluk
G,
Tutar
E,
Bayrak
A,
Volkan
B,
Akyon
Y,
Celikel
C,
Ertem
D.
Sequential
therapy
versus
standard
triple
therapy
for
H.
pylori
eradication
in
children:
any
advantage
in
clarithromycin-resistant
strains?
European
journal
of
gastroenterology
&
hepatology
2014;
26:
1202-8.
[44]
Greenberg
ER,
Anderson
GL,
Morgan
DR,
Torres
J,
Chey
WD,
Bravo
LE,
Dominguez
RL,
Ferreccio
C,
Herrero
R,
Lazcano-Ponce
EC.
14-day
triple,
5-day
concomitant,
and
10-day
sequential
therapies
for
H.
pylori
infection
in
seven
Latin
American
sites:
a
randomised
trial.
The
Lancet
2011;
378:
507-14.
[45]
Khaleghi
S,
Naghibi
S,
Naghibi
S.
Comparison
of
sequential
and
routine
four
drugs
therapeutic
regiments
in
H.
pylori
eradication.
Journal
of
Gorgan
University
of
Medical
Sciences
2013;
15:
1-6.
[46]
Kaboli
SA,
Zojaji
H,
Mirsattari
D,
Talaie
R,
Derakhshan
F,
Zali
MR,
Sheikhvatan
M.
Effect
of
addition
of
vitamin
C
to
clarithromycin-amoxicillin-omeprazol
triple
regimen
on
H.
pylori
eradication.
Acta
gastro-enterologica
Belgica
2008;
72:
222-4.
[47]
Zullo
A,
Hassan
C,
De
Francesco
V,
Lorenzetti
R,
Marignani
M,
Angeletti
S,
Ierardi
E,
Morini
S.
A
third-line
levofloxacin-based
rescue
therapy
for
H.
pylori
eradication.
Digestive
and
liver
disease
2003;
35:
232-6.
[48]
Bastos
J,
Peleteiro
B,
Barros
R,
Alves
L,
Severo
M,
Fátima
Pina
M,
Pinto
H,
Carvalho
S,
Marinho
A,
Guimarães
JT.
Sociodemographic
determinants
of
prevalence
and
incidence
of
H.
pylori
infection
in
Portuguese
adults.
Helicobacter
2013;
18:
413-22.
[49]
Benajah
DA,
Lahbabi
M,
Alaoui
S,
El
Rhazi
K,
El
Abkari
M,
Nejjari
C,
Amarti
A,
Bennani
B,
Mahmoud
M,
Ibrahimi
SA.
Prevalence
of
H.
pylori
and
its
recurrence
after
successful
eradication
in
a
developing
nation
(Morocco).
Clinics
and
research
in
hepatology
and
gastroenterology
2013;
37:
519-26.
[50]
Ozaydin
N,
Turkyilmaz
SA,
Cali
S.
Prevalence
and
risk
factors
of
H.
pylori
in
Turkey:
a
nationally-representative,
cross-sectional,
screening
with
the
13
C-Urea
breath
test.
BMC
Public
Health
2013;
13:
1.
[51]
Ghasemi-Kebria
F,
Ghaemi
E,
Azadfar
S,
Roshandel
G.
Epidemiology
of
H.
pylori
infection
among
Iranian
children.
Arab
Journal
of
Gastroenterology
2013;
14:
169-72.
[52]
Mana
F,
Vandebosch
S,
Deyi
VM,
Haentjens
P,
Urbain
D.
Prevalence
of
and
risk
factors
for
H.
pylori
infection
in
healthy
children
and
young
adults
in
Belgium
anno
2010/2011.
Acta
Gastro
Enterol
Belg
2013;
76:
381-5.
[53]
Tamadon
MR,
Zahmatkesh
M.
Helicobacter
pylori
in
patients
with
chronic
renal
failure;
a
new
update.
Geriatr
Persia.
2017;
1(1):e02.

