|
|
 |
| ............................................................. |
|
|
| ........................................................ |
| From
the Editor |

|
Editorial
A. Abyad (Chief Editor) |
|
|
|
|
........................................................ |
Original
Contribution / Clinical Investigation
|






|
Serum
level of ionized calcium in patients with migraine
during a migraine attack and times without migraine
[pdf version]
Mojtaba Kazemi, Sajad Emami, Aida Bahman, Mahshid
Zareian, Abdolreza Sotoodeh Jahromi, Somayehsadat
Talebnia Jahromi, Hassan Zabetian, Ali Golestan,
Abdolhossein Madani
Comparative
study of vocational rehabilitation among governmental
and private sectors on employment of disabled
persons
[pdf
version]
Mansour Nazari Chafjiri
A Survey of Interurban Taxi Drivers'driving
Behaviors across Kermanshah, Iran, in 2015
[pdf
version]
Reza Pourmirza Kalhori, Azadeh Foroughinia,
Arash Ziapour
Comparison of
Standard Triple Therapy Regimen with Sequential
Therapy Regimen Containing Levofloxacin Used
for The Eradication of Helicobacter Pylori in
Patients with Gastrointestinal Infection Caused
by Helicobacter Pylori
[pdf version]
Masoud Hafizi, Mohammad Hadi Shafigh Ardestani,
Mohammad Reza Tamadon,
Kian Kavehzadeh, Masoud Amiri
Risk Factors
of Resistance to Intravenous Immunoglobulin
in patients with Kawasaki: A Cross-Sectional
Study over a 10 Year Period (2006-2016)
[pdf version]
Fariba Tarhani, Azadeh Jafrasteh, Mahshid Garmsiri,
Shabnam Dalvand
Evaluation of hematological indices of workers
exposed to benzene
[pdf version]
Behnaz Salehiforouz, Akbar Vahdati, Ali Akbar
Malekirad, Mohammad Amin Edalatmanesh
|
........................................................
Community Research
|






|
The
Effect of Internet Usage on Relations between
Members of the Iranian Family in Tehran City
[pdf version]
Lida Norouzi, Anahita Arbabi, Maryam Jamali
Investigate
the Relationship between Information Technology
and Employees' Productivity with Mediating Role
of Knowledge Management (Case study: Imam Reza
Hospital of Sirjan)
[pdf
version]
Hamid Shahdadi, Abbas Yazdanpenah, Abbas Ghavam
Pre-competition anxiety score among Elite Boy
Swimmers in Iran
[pdf version]
Asghar Nikseresht, Amir-Abbas Yabande, Karamatollah
Rahmanian, Abdolreza Sotoodeh Jahromi
Assessment
of the Presence or Absence of Palmaris Longus
and the Fifth Superficial Flexor Tendon in the
Iranian Population: Are these tendons evolutionary?
[pdf
version]
Afshin Ahmadzadeh Heshmati, Ali Karbalaeikhani,
Alireza Saied, Mohsen Rouhani,
Mahsa Aboei, Farzad Abroud, Elahe Havoshk
Moral
challenges in the provision of care for Infant
and Family: a qualitative study
[pdf
version]
Marzieh Zahabi, Narges Sadeghi
A
Study of the Effects of Factors Related to Food
Consumption in Health Workers of Najaf Abad-based
Healthcare Centers, Isfahan, Iran
[pdf
version]
Fereshteh Sarmadi
|
........................................................
Review Article
........................................................
Clinical
Research and Methods
........................................................
Office
Research
........................................................
Basic
and Laboratory Research
|



|
The
effects of Matricaria Chamomilla extract during
neonatal period of rats on pituitary-gonadal
hormone axis and changes in testicular tissue
of male progenies
[pdf
version]
Safieh Golkhani, Akbar Vahdati , Mehrdad Modaresi,
Mohammad Amin Edalatmanesh
In
Vitro Effects of Ascorbic Acid on Corneal Collagen
Cross-Linking in Keratoconus
[pdf
version]
Nasrin Aghaei , Shahrokh Ramin, Abbas Aghaei
, Sayed Mehdi Tabatabaei, Mohammd Aghazadeh
Amiri
Investigating
the prenatal exposure of hydro-alcoholic extract
of ginger on the function of Pituitary - Gonad
axis in male mature offspring rats
[pdf version]
Nasim Zamani, Ebrahim Hosseini, Mehrdad Modaresi,
Abdallah Ghasemi Pirbalouti
|
|
Chief
Editor -
Abdulrazak
Abyad
MD, MPH, MBA, AGSF, AFCHSE
.........................................................
Editorial
Office -
Abyad Medical Center & Middle East Longevity
Institute
Azmi Street, Abdo Center,
PO BOX 618
Tripoli, Lebanon
Phone: (961) 6-443684
Fax: (961) 6-443685
Email:
aabyad@cyberia.net.lb
.........................................................
Publisher
-
Lesley
Pocock
medi+WORLD International
11 Colston Avenue,
Sherbrooke 3789
AUSTRALIA
Phone: +61 (3) 9005 9847
Fax: +61 (3) 9012 5857
Email:
lesleypocock@mediworld.com.au
.........................................................
Editorial
Enquiries -
abyad@cyberia.net.lb
.........................................................
Advertising
Enquiries -
lesleypocock@mediworld.com.au
.........................................................
While all
efforts have been made to ensure the accuracy
of the information in this journal, opinions
expressed are those of the authors and do not
necessarily reflect the views of The Publishers,
Editor or the Editorial Board. The publishers,
Editor and Editorial Board cannot be held responsible
for errors or any consequences arising from
the use of information contained in this journal;
or the views and opinions expressed. Publication
of any advertisements does not constitute any
endorsement by the Publishers and Editors of
the product advertised.
The contents
of this journal are copyright. Apart from any
fair dealing for purposes of private study,
research, criticism or review, as permitted
under the Australian Copyright Act, no part
of this program may be reproduced without the
permission of the publisher.
|
|
|
| August 2017 -
Volume 15, Issue 6 |
|
|
Restoration of Let-7:
a possible approach for increased sensitivity
to paclitaxel in ovarian cancer
Mohammad-Reza Mahmoudian-Sani (1)
Ameneh Mehri-Ghahfarrokhi (2)
Ali Shojaeian (3)
Majid Asadi-Samani (4)
(1) Research Center for Molecular Medicine,
Hamadan University of Medical Sciences, Hamadan,
Iran;
(2) Cellular and Molecular Research Center,
Basic Health Sciences Institute, Shahrekord
University of Medical Sciences, Shahrekord,
Iran;
(3) Medical Plants Research Center, Basic Health
Sciences Institute, Shahrekord University of
Medical Sciences, Shahrekord, Iran;
(4) Students Research Committee, Shahrekord
University of Medical Sciences, Shahrekord,
Iran.
Correspondence:
Majid Asadi-Samani,Medical Plants Research Center,
Basic Health Sciences Institute, Shahrekord
University of Medical Sciences, Rahmatiyeh,
Shahrekord, Iran
Email: biology_2011@yahoo.com
|
Abstract
Ovarian
cancer is one of the common cancers of
the female reproductive system. Paclitaxel
is the first-line treatment of ovarian
cancer and the second-line treatment of
advanced ovarian cancer. Unfortunately,
many patients cannot be treated because
of drug resistance. miRNAs comprise a
group of small non-coding RNAs 18-25 nucleotides
in length that specifically interact with
their own mRNAs. Many miRNAs that have
so far been identified play a role in
cancer. miRNAs regulate formation of cancer
stem cells (CSCc) and drug resistance-associated
epithelial-mesenchymal transition (EMT)
phenotype. The let-7 miRNA is a founding
member of the miRNA family and is conserved
in invertebrates and vertebrates. In this
review paper we have tried to describe
a possible approach for increased sensitivity
to paclitaxel in ovarian cancer by restoration
of Let-7. In addition to suppressing tumorigenic
activities and negatively regulating a
number of oncogenes (Kras-Hras-HMGA2-c-myc-BF2),
let-7 affects the main regulators of cell
cycle, cell differentiation, and apoptosis
pathway. Let-7 via RNA decomposition of
the IMP-1 gene increased sensitivity to
paclitaxel drug. Various compounds such
as Isoflavone specifically can affect
expression of Let-7.
Although let-7 is a potential therapeutic
target for therapy resistant ovarian cancer,
further studies should be conducted to
investigate clinical use of let-7 to treat
or suppress ovarian cancer.
Key words:
miRNA, Ovarian cancer, Drug resistance,
Let-7
|
Today cancer as a deadly disease causes many
problems for all of the people in the world.
Ovarian cancer is one of the common cancers
of the female reproductive system and one of
the most life-threatening cancers such that
it is the cause of over 50% of deaths due to
gynecological cancers. At early stages, ovarian
cancer is asymptomatic or its symptoms may be
so vague that they cannot be detected by physician
or the patient (1). There are many drugs for
prevention and treatment of cancers such as
ovarian cancer. Natural products such as medicinal
plants have been used as one of the main resources
for production of anticancer drugs (2-7). Paclitaxel
as a natural compound is the first-line treatment
of ovarian cancer and the second-line treatment
of advanced ovarian cancer that prevents microtubule
depolymerization in the process of cell proliferation.
Hence, paclitaxel inhibits cell cycle. If ovarian
cancer is diagnosed at early stages, treatment
consists of surgery and chemotherapy. At advanced
stages, chemotherapy is started as well, but
unfortunately treatment may not be successful
in many cases because of drug resistance (8)
such that following surgery, combination chemotherapy
(paclitaxel+carboplatin) is also used with an
80% response rate.
However, in most patients, unfortunately, recurrent
cancer develops and the disease becomes resistant
to chemotherapy after 18 months. Currently,
the cell line NCI/ADR-RES, which has become
resistant to paclitaxel, and the cell line OVCAR8,
as control, are used to investigate resistance
to ovarian cancer drugs in vitro. In this review
paper we have tried to describe a possible approach
for increased sensitivity to paclitaxel in ovarian
cancer by restoration of Let-7.
miRNAs comprise a group of small non-coding
RNAs approximately 18-25 nucleotides in length
that cause destruction of mRNA and inhibition
of its translation. miRNA genes comprise approximately
1% of the genome of different species. Each
miRNA gene has hundreds of target genes. Over
2500 miRNAs have been identified in the human
genome that regulate 30% of protein-coding genes.
Most of these small regulatory molecules that
were first identified in 1983 are located on
Chromosomal fragile regions that are predisposed
to removal, addition, chromosomal replacements,
and epigenetic changes in different diseases
such as cancer. miRNAs target several genes
simultaneously such that the number of target
genes may exceed 100 (9, 10). Since 2002, disruption
of miRNA regulation has been found to be associated
with cancer (11, 12).
miRNA
in
the
nucleus
transcribes
the
gene
and
produces
primary
miRNA
(pri-miRNA).
Then,
Drosha
creates
a
precursor
called
pre-miRNA
under
RNaseIII
(endonuclease),
and
pre-miRNA
is
transferred
to
cytoplasm
by
exportin-5.
This
molecule
is
cleaved
by
an
enzyme
called
Dicer
and
produces
a
double-stranded
sequence
20-22
nucleotides
in
length.
One
of
the
strands
is
degraded
and
the
miRNA’s,
another
strand,
is
loaded
into
RNA-induced
silencing
complex
(RISC).
This
active
complex
targets
the
mRNA
of
interest
and
binds
to
the
end
of
3`-UTR
mRNA,
and
exerts
inhibitory
effect.
miRNA
induces
its
effect
in
regulating
gene
expression
through
inhibiting
the
protein
translation
and
decomposing
the
target
mRNA
(13).
Many
miRNAs
that
have
so
far
been
identified
play
a
role
in
cancer.
Comparing
tumor
tissues
with
healthy
tissues
has
indicated
that
miRNAs
are
located
at
fragile
sites
of
the
human
genome
and
are
likely
to
face
gene
deletion
or
duplication
at
chromosomal
rearrangement.
Besides
that,
it
is
possible
that
epigenetic
mechanisms
lead
to
inappropriate
expression
of
miRNA
genes
and
cause
abnormal
expression
of
miRNAs
in
tumor
tissues
leading
to
numerous
changes
in
regulation
of
the
target
miRNA
expression.
Many
miRNAs
play
no
part
in
development
of
cancer.
In
contrast,
certain
miRNAs
play
an
oncogenic
role
in
cancer
phenotype,
and
dysregulation
of
these
miRNAs
has
been
reported
in
a
wide
spectrum
of
cancers
(14).
Oncogenic
miRNAs
include
miR-10b,
miR-155,
miR-21,
and
miR-17-92
(15),
and
out
of
repressive
miRNAs,
miR-26a,
miR-335,
and
members
of
the
families
let-7
and
miR-34
can
be
mentioned.
Different
miRNAs
affect
different
stages
of
cancer.
For
example,
miR-10b
regulates
metastasis
and
is
highly
expressed
in
advanced
malignancies.
Inhibition
of
miR-10b
can
prevent
metastasis
of
cancer
cells
but
has
no
effect
on
already
developed
metastases.
Expression
of
miR-335
can
prevent
metastasis
but
cannot
prevent
proliferation
of
tumor
cells
and
has
no
effect
on
cell
apoptosis
rate.
However,
some
miRNAs
can
prevent
proliferation
of
tumor
cells
and
metastasis
(16).
miRNA
expression
has
been
reported
to
change
(increase
or
decrease)
in
different
human
cancers
(17,
18)
.
| miRNA
and
chemotherapy
resistance |
Recently,
some
studies
have
found
miRNA
and
chemotherapy
resistance
to
be
associated
(19,
20).
In
recent
years,
considerable
advancements
have
been
made
to
figure
out
drug
resistance
mechanism
in
ovarian
cancer
consisting
of
drug
efflux,
changes
in
DNA
repair
pathway,
apoptosis
suppression,
and
epithelial-mesenchymal
transition
and
cancer
stem
cells.
However,
more
effective
therapeutic
purposes
are
still
needed
to
improve
overall
survival
rate
and
therapeutic
strategies
for
ovarian
cancer
patients.
miRNAs
play
a
critical
role
in
cell
processes
such
as
cellular
differentiation,
proliferation,
and
apoptosis.
The
recent
discovery
of
miRNAs
in
cancer
has
offered
new
paths
for
research
on
basic
mechanisms
of
response
to
chemotherapy.
Besides
that,
several
studies
have
demonstrated
that
certain
miRNAs
such
as
let-7
and
miR-34a
can
affect
response
to
chemotherapy
in
different
types
of
tumors
including
ovarian
cancer.
Use
of
miRNAs
to
overcome
resistance
to
treatment
is
being
studied.
This
study
investigated
the
role
of
let-7
in
overcoming
resistance
to
treatment
through
several
molecular
mechanisms
and
with
emphasis
on
potential
therapeutic
uses.
Click
her
for
Figure
1
Figure
1:
Biogenesis
of
miRNA:
miRNA
biogenesis
is
a
multistep
process.
First,
miRNA
genes
are
transcribed
by
RNA
polymerase
II
in
the
nucleus.
The
resulting
primary
transcript
is
cleaved
by
Drosha
and
DGCR8
to
produce
pre-miRNA.
After
exportin-5-
and
RanGTP-mediated
transport
to
the
cytoplasm,
the
pre-miRNA
undergoes
its
final
processing
step,
which
consists
of
Dicer-dependent
cleavage
just
below
the
stem
loop
to
produce
a
duplex
molecule.
The
duplex
is
then
separated
and
usually
one
strand
is
selected
as
the
mature
miRNA
and
directed
to
target-specific
mRNAs
Let-7
is
from
a
13-member
family
localized
on
nine
different
chromosomes.
An
association
between
let-7
and
drug
resistance
has
been
demonstrated.
Recent
studies
have
demonstrated
that
let-7
specifically
affects
3-Urt-BCL-XL
in
hepatocellular
cell
line
and
let-7
high
expression
makes
the
cells
susceptible
to
sorafenib
(21).
Since
let-7
expression
has
been
reported
to
decrease
in
many
cancers,
the
changes
in
the
expression
of
this
miRNA
are
likely
to
be
associated
with
chemotherapy
resistance,
but
the
data
are
scant
in
this
field.
Igf2
mRNA
binding
protein1
(IMP-1)
is
a
drug
resistance-associated
protein
and
it
has
recently
been
demonstrated
that
IMP-1
level
is
associated
with
let-7
level.
In
fact,
let-7
negatively
regulates
IMP-1
which
in
turn
exerts
protective
effect
on
multi-drug
resistance
(MDR-1).
Measuring
let-7
in
different
cell
lines
indicated
that
the
members
of
this
family
were
co-regulated
and
co-expressed.
Let-7
expression
has
been
demonstrated
to
decrease
before
and
after
treatment
with
chemotherapy
drugs,
which
is
associated
with
increased
production
of
IMP-1
and
MDR-1.
| Molecular
mechanisms
of
chemotherapy
resistance
in
cancer |
Chemotherapy
resistance
develops
molecularly
via
two
pathways
consisting
of
de
novo
or
internal
pathway
through
CSCs,
and
external
or
acquired
pathways
including
genetic
and
epigenetic
changes.
However,
the
precise
mechanisms
of
chemotherapy
resistance
generally
have
not
yet
been
identified.
In
de
novo
pathway,
limited
drug
absorption,
increased
efflux,
and
activated
detoxification
and
in
the
second
pathway,
epigenetic
changes
such
as
DNA
methylation-histone
modification
and
mRNA
regulation
play
part
in
drug
resistance.
For
example,
in
colorectal
cancer,
the
transcription
factors
AP2E
and
DKK4
undergo
methylation
changes
that
cause
them
to
become
resistant
to
fluorouracil.
In
ovarian
cancer,
the
gene
MLH1-TAP73
is
hypermethylated
and
predisposed
to
acquiring
resistance
to
d-azacitidine-hydralazine.
miRNA
deregulation
has
been
demonstrated
to
be
associated
with
cancer
drug
resistance.
For
example,
in
breast
cancer,
increased
expression
of
miR-21
leads
to
trastuzumab
resistance.
In
case
of
resistance
to
cisplatin
in
ovarian
cancer,
the
expression
rates
of
miR
376
and
miR-214
increase
and
therefore
it
is
necessary
to
study
miRNAs
so
Chemotherapy
resistance
develops
molecularly
via
two
pathways
consisting
of
de
novo
or
internal
pathway
through
CSCs,
and
external
or
acquired
pathways
including
genetic
and
epigenetic
changes.
However,
the
precise
mechanisms
of
chemotherapy
resistance
generally
have
not
yet
been
identified.
In
de
novo
pathway,
limited
drug
absorption,
increased
efflux,
and
activated
detoxification
and
in
the
second
pathway,
epigenetic
changes
such
as
DNA
methylation-histone
modification
and
mRNA
regulation
play
part
in
drug
resistance.
For
example,
in
colorectal
cancer,
the
transcription
factors
AP2E
and
DKK4
undergo
methylation
changes
that
cause
them
to
become
resistant
to
fluorouracil.
In
ovarian
cancer,
the
gene
MLH1-TAP73
is
hypermethylated
and
predisposed
to
acquiring
resistance
to
d-azacitidine-hydralazine.
miRNA
deregulation
has
been
demonstrated
to
be
associated
with
cancer
drug
resistance.
For
example,
in
breast
cancer,
increased
expression
of
miR-21
leads
to
trastuzumab
resistance.
In
case
of
resistance
to
cisplatin
in
ovarian
cancer,
the
expression
rates
of
miR
376
and
miR-214
increase
and
therefore
it
is
necessary
to
study
miRNAs
so
that
molecular
mechanisms
of
cancer
drug
resistance
may
be
known
(22).
Recent
studies
have
demonstrated
that
acquired
resistance
(genetic
and
epigenetic
changes)
is
the
main
reason
for
drug
resistance
in
ovarian
cancer
but
further
studies
are
needed
to
identify
signaling
pathways
that
are
regulated
by
miRNA,
such
as
NOTCH-FOXM1,
so
that
valuable
information
about
drug
resistance
can
be
obtained
(22).
| Potential
mechanisms
of
let-7
action
on
ovarian
cancer |
Let-7
regulates
CSCs
and
EMT
formation
which
is
associated
with
drug
resistance
(23,
24).
Let-7
exerts
regulatory
effects
on
p53
(25).
Let-7
negatively
regulates
MDR
and
indeed
exerts
effect
on
MDR1
indirectly
through
IMP1.
Let-7
causes
decomposition
of
mRNA
related
to
IMP-1
which
is
both
a
target
of
let-7
and
inhibits
endolithic
activity
of
MDR1
(26).
In
experimental
studies,
silencing
the
gene
EZH2
has
been
demonstrated
to
cause
decrease
in
cell
proliferation,
M-G2
arrest,
and
cell
drug
susceptibility.
Increased
expression
of
let-7
causes
the
expression
of
the
gene
EZH2
to
decrease
but
it
has
not
yet
been
discovered
how
this
occurs.
Let-7a,
let-7b,
and
let-7c
exert
inhibitory
effects
on
EZH2.
Let-7
can
also
exert
inhibitory
or
down-regulatory
effect
on
CCND1.
CCND1
is
a
member
of
the
family
of
cyclins
that
affects
cell
cycle
and
its
expression
in
tumors
increases
in
cisplatin
resistance
(26).
Studies
have
demonstrated
that
the
expression
of
the
common
miRNAs
that
exist
in
most
paclitaxel-resistant
cell
lines
is
associated
with
ovarian
cancer.
These
miRNAs
include
miR:
pre218-
let-7e-130a-130b-pre204-0c-335-106-pre106,
and
let-7
(27).
MS-PCR
results
have
indicated
that
in
chemotherapy
drug-resistant
cell
lines,
let-7-related
CPG
hypermethylation
occurs
in
DNA
in
most
cancers
including
ovarian
cancer,
and
since
one
of
the
mechanisms
of
disrupted
expression
(deregulation)
and
decreased
expression
of
let-7
is
hypermethylation,
then
hypermethylation
is
likely
to
occur
in
ovarian
cancer
as
well.
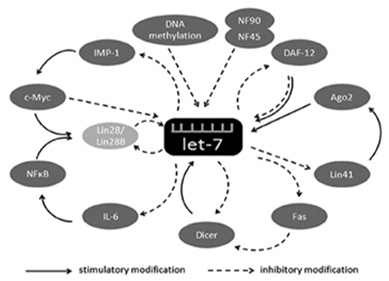
Figure
2:
illustrates
the
mechanisms
and
factors
that
affect
let-7.
IMP-1
plays
a
role
in
drug
resistance
and
inhibits
its
let-7.
DNA
methylation
exerts
inhibitory
effect
on
let-7
(28).
| Flavonoid’s
effect
on
let-7
expression |
Recently,
nature-based
compounds
such
as
isoflavone
and
DIM
have
been
demonstrated
to
affect
the
expression
of
miRNAs
including
let-7
and
can
induce
its
expression;
therefore,
flavonoid’s
effect
on
paclitaxel
transporters
can
be
investigated
in
a
resistant
cell
line,
termed
NCI/ADR-RES.
Flavonoids
affects
miR-21
expression
and
increases
its
production,
and
causes
increase
in
production
of
the
molecules
PTEN-PDCD4-RECK
that
progress
the
cell
mainly
toward
apoptosis.
Although
no
study
has
yet
reported
clinical
use
of
these
compounds,
clinical
trials
at
different
phases
are
being
conducted
(25).
Synthetic
and/or
nature-based
compounds
derived
from
plant
flavonoids
mainly
target
malignant
cells.
In
neuroblastoma,
the
flavonoid
and
retinoid
compound,
called
cyclincyc,
can
exert
effect
on
miRNA
with
oncogenic
role
and
miRNA
with
tumor-suppressing
role
(23).
Since
synthetic
let-7
has
limited
use
and
is
easily
degraded,
use
of
flavonoids
to
increase
let-7
expression
seems
appropriate.
Introduction
of
miRNAs
and
their
role
represents
a
new
level
of
controlling
gene
expression.
Studies
have
demonstrated
that
disrupted
regulation
of
miRNAs
can
be
an
important
stage
of
progression
in
most
cancers.
Dysregulation
of
miRNAs
can
be
due
to
genetic
mutations
or
regulation
at
transcription
level
which
are
important
mechanisms
of
increased
expression
of
the
target
genes
causing
tumorigenesis.
miRNA-based
treatments
are
based
on
two
bases;
use
of
mimics
miRNAs
that
is
mainly
conducted
by
miRNA
replacement
therapy
and
causes
the
expression
levels
of
tumor-suppressing
miRNAs
(undergoing
decrease
in
expression)
to
reach
normal
levels.
The
second
approach
is
use
of
their
antagonists
which
are
mainly
used
to
inhibit
function
of
oncogenic
miRNAs.
A
drug
called
AS1411
is
from
a
group
of
compounds
called
G-rich
aptamer.
This
drug
acts
via
blocking
production
of
oncogenic
miRNAs
in
the
cell
whose
expression
levels
increase
in
cancer.
AS1411
inhibits
a
protein
called
nucleolin
that
plays
an
important
role
in
miRNA
maturation
(29).
In
studies
miRNA
microarray,
decreased
expression
of
miR30C-miR130a-let7
was
demonstrated
to
cause
paclitaxel
resistance
and
cisplatin.
Moreover,
let-7
inhibits
certain
oncogenic
proteins
such
as
Kras-Hras-HMGA2-c-myc-NF2.
Studies
have
demonstrated
that
the
removal
rate
of
DNA
in
ovarian
cancer
is
over
44%
for
Let-7a-3
and
let-7b
(30).
In
the
near
future,
use
of
nanoparticle
technology,
particularly
in
cancer
drug
resistance,
will
facilitate
use
of
let-7
and
other
miRNAs
in
treatment.
Let-7
nanoparticle
is
being
used
In
vitro
(31).
Taken
together,
regarding
the
potential
role
of
let-7
in
paclitaxel
resistance
in
ovarian
cancer
and
that
let-7
can
suppress
the
expression
of
the
genes
involved
in
this
cancer,
let-7
has
attracted
attention
as
a
potential
therapeutic
target
in
therapy
resistant
ovarian
cancer.
In
addition
to
suppressing
tumorigenic
activities
and
negatively
regulating
a
number
of
oncogenes
(Kras-Hras-HMGA2-c-myc-BF2),
let-7
affects
the
main
regulators
of
cell
cycle,
cell
differentiation,
and
apoptosis
pathway.
Therefore,
let-7
can
be
used
to
inhibit
the
expression
of
these
genes
and
therefore
therapy
resistant
cancer.
Regarding
the
findings
of
recent
studies,
we
can
use
increase
in
expression
of
let-7
using
medicinal
plants,
or
mimic
production
of
it
as
a
synthetic
and
transporting
it
into
the
cell
to
enhance
treatment
and
control
the
growth
of
ovarian
cancer,
and
mimic
let-7
most
probably
can
be
used
as
an
adjuvant
drug
in
the
treatment
protocol
for
patients
with
paclitaxel
resistance.
However,
this
issue
requires
further
investigation.
Increase
in
Let-7
expression
is
expected
to
serve
as
an
effective
treatment
for
therapy
resistant
cancer.
Although
acceptable
advancements
have
been
made
to
figure
out
regulation
of
let-7
synthesis
and
role
in
signalling
pathways,
its
regulation
in
cancer
and
normal
cells,
and
mechanism
of
cell
proliferation
control
and
cell
survival
need
further
investigation.
Further
studies
are
needed
to
use
let-7
in
clinical
settings
to
treat
or
suppress
therapy
resistant
cancer.
1.
Cho
KR,
Shih
Ie
M.
Ovarian
cancer.
Annual
review
of
pathology.
2009;4:287-313.
2.
Asadi-Samani
M,
Kooti
W,
Aslani
E,
Shirzad
H.
A
systematic
review
of
Iran’s
medicinal
plants
with
anticancer
effects.
Journal
of
evidence-based
complementary
&
alternative
medicine.
2016;21(2):143-53.
3.
Kooti
W,
Servatyari
K,
Behzadifar
M,
Asadi-Samani
M,
Sadeghi
F,
Nouri
B,
et
al.
Effective
Medicinal
Plant
in
Cancer
Treatment,
Part
2.
Journal
of
evidence-based
complementary
&
alternative
medicine.
2017:2156587217696927.
4.
Gholamian-Dehkordi
N,
Luther
T,
Asadi-Samani
M,
Mahmoudian-Sani
MR.
An
overview
on
natural
antioxidants
for
oxidative
stress
reduction
in
cancers;
a
systematic
review.
Immunopathologia
Persa.
2017;3(2):e12.
5.
Afkhami-Ardakani
M,
Hassanzadeh
S,
Shahrooz
R,
Asadi-Samani
M,
Latifi
M,
Luther
T.
Phytotherapy
and
phytopharmacology
for
reduction
of
cyclophosphamide-induced
toxicity
in
the
male
urinary
system.
Journal
of
Renal
Injury
Prevention
2017;6(3):164-70.
6.
Rahimifard
M,
Sadeghi
F,
Asadi-Samani
M,
Nejati-Koshki
K.
Effect
of
quercetin
on
secretion
and
gene
expression
of
leptin
in
breast
cancer.
Journal
of
Traditional
Chinese
Medicine.
2017;37(3):321-5.
7.
Chaleshtori
JS,
Soreshjani
EH,
Reisi
F,
TabaTabaiefar
MA,
Asadi-Samani
M,
Navid
Z,
et
al.
Damage
intensity
of
carvacrol
on
prostatic
cancer
cells
lineDu145
and
molecular
dynamic
simulation
of
it
effect
on
apoptotic
factors.
International
Journal
of
PharmTech
Research.
2016;9(6):261-73.
8.
Kim
A,
Ueda
Y,
Naka
T,
Enomoto
T.
Therapeutic
strategies
in
epithelial
ovarian
cancer.
Journal
of
experimental
&
clinical
cancer
research
2012;31:14.
9.
Heneghan
HM,
Miller
N,
Kerin
MJ.
MiRNAs
as
biomarkers
and
therapeutic
targets
in
cancer.
Current
opinion
in
pharmacology.
2010;10(5):543-50.
10.
Slabý
O,
Svoboda
M,
Fabian
P,
Svoboda
M,
Garajová
I,
Šachlová
M,
et
al.
Association
of
miR-21,
miR-31,
miR-143,
miR-145
and
let-7a-1
levels
with
histopathologic
features
of
colorectal
cancer.
European
Journal
of
Cancer.
2007;
5(4):
78-79.
11.
Calin
GA,
Dumitru
CD,
Shimizu
M,
Bichi
R,
Zupo
S,
Noch
E,
et
al.
Frequent
deletions
and
down-regulation
of
micro-RNA
genes
miR15
and
miR16
at
13q14
in
chronic
lymphocytic
leukemia.
Proceedings
of
the
National
Academy
of
Sciences.
2002;99(24):15524-9.
12.
Mahmoudian-sani
M-R,
Mehri-Ghahfarrokhi
A,
Asadi-Samani
M,
Mobini
G-R.
Serum
miRNAs
as
Biomarkers
for
the
Diagnosis
and
Prognosis
of
Thyroid
Cancer:
A
Comprehensive
Review
of
the
Literature.
European
Thyroid
Journal.
2017:In
Press.
13.
Bhatt
K,
Mi
Q-S,
Dong
Z.
microRNAs
in
kidneys:
biogenesis,
regulation,
and
pathophysiological
roles.
American
Journal
of
Physiology-Renal
Physiology.
2011;300(3):F602-F10.
14.
Orellana
EA,
Kasinski
AL.
MicroRNAs
in
cancer:
a
historical
perspective
on
the
path
from
discovery
to
therapy.
Cancers.
2015;7(3):1388-405.
15.
Medina
PP,
Nolde
M,
Slack
FJ.
OncomiR
addiction
in
an
in
vivo
model
of
microRNA-21-induced
pre-B-cell
lymphoma.
Nature.
2010;467(7311):86-90.
16.
Reinhart
BJ,
Slack
FJ,
Basson
M,
Pasquinelli
AE,
Bettinger
JC,
Rougvie
AE,
et
al.
The
21-nucleotide
let-7
RNA
regulates
developmental
timing
in
Caenorhabditis
elegans.
Nature.
2000;403(6772):901-6.
17.
Xie
Q,
Chen
X,
Lu
F,
Zhang
T,
Hao
M,
Wang
Y,
et
al.
Aberrant
expression
of
microRNA
155
may
accelerate
cell
proliferation
by
targeting
sex-determining
region
Y
box
6
in
hepatocellular
carcinoma.
Cancer.
2012;118(9):2431-42.
18.
Zhang
Y,
Wei
W,
Cheng
N,
Wang
K,
Li
B,
Jiang
X,
et
al.
Hepatitis
C
virus-induced
up-regulation
of
microRNA-155
promotes
hepatocarcinogenesis
by
activating
Wnt
signaling.
Hepatology
(Baltimore,
Md).
2012;56(5):1631-40.
19.
Wang
F,
Liu
M,
Li
X,
Tang
H.
MiR-214
reduces
cell
survival
and
enhances
cisplatin-induced
cytotoxicity
via
down-regulation
of
Bcl2l2
in
cervical
cancer
cells.
FEBS
letters.
2013;587(5):488-95.
20.
Chan
JK,
Blansit
K,
Kiet
T,
Sherman
A,
Wong
G,
Earle
C,
et
al.
The
inhibition
of
miR-21
promotes
apoptosis
and
chemosensitivity
in
ovarian
cancer.
Gynecologic
oncology.
2014;132(3):739-44.
21.
Ray
SK.
Emerging
roles
of
microRNAs
in
malignant
neuroblastoma.
Clinical
&
Experimental
Pharmacology.
2013;2013.
22.
Cai
J,
Yang
C,
Yang
Q,
Ding
H,
Jia
J,
Guo
J,
et
al.
Deregulation
of
let-7e
in
epithelial
ovarian
cancer
promotes
the
development
of
resistance
to
cisplatin.
Oncogenesis.
2013;2(10):e75.
23.
Peter
ME.
Regulating
cancer
stem
cells
the
miR
way.
Cell
stem
cell.
2010;6(1):4-6.
24.
Zheng
T,
Wang
J,
Chen
X,
Liu
L.
Role
of
microRNA
in
anticancer
drug
resistance.
International
journal
of
cancer.
2010;126(1):2-10.
25.
Sarkar
FH,
Li
Y,
Wang
Z,
Kong
D,
Ali
S.
Implication
of
microRNAs
in
drug
resistance
for
designing
novel
cancer
therapy.
Drug
Resistance
Updates.
2010;13(3):57-66.
26.
Xie
Z,
Cao
L,
Zhang
J.
miR-21
modulates
paclitaxel
sensitivity
and
hypoxia-inducible
factor-1
expression
in
human
ovarian
cancer
cells.
Oncology
letters.
2013;6(3):795-800.
27.
Sorrentino
A,
Liu
C-G,
Addario
A,
Peschle
C,
Scambia
G,
Ferlini
C.
Role
of
microRNAs
in
drug-resistant
ovarian
cancer
cells.
Gynecologic
oncology.
2008;111(3):478-86.
28.
Wang
X,
Cao
L,
Wang
Y,
Wang
X,
Liu
N,
You
Y.
Regulation
of
let-7
and
its
target
oncogenes
(Review).
Oncology
letters.
2012;3(5):955-60.
29.
Kutanzi
KR,
Yurchenko
OV,
Beland
FA,
Vasyl
F
C,
Pogribny
IP.
MicroRNA-mediated
drug
resistance
in
breast
cancer.
Clinical
epigenetics.
2011;2(2):171.
30.
Wang
Y,
Hu
X,
Greshock
J,
Shen
L,
Yang
X,
Shao
Z,
et
al.
Genomic
DNA
copy-number
alterations
of
the
let-7
family
in
human
cancers.
PloS
one.
2012;7(9):e44399.
31.
Gadducci
A,
Sergiampietri
C,
Lanfredini
N,
Guiggi
I.
Micro-RNAs
and
ovarian
cancer:
the
state
of
art
and
perspectives
of
clinical
research.
Gynecological
Endocrinology.
2014;30(4):266-71.
|
|
.................................................................................................................

|
| |
|

