In
total,
52
patients
with
final
Kawasaki
diagnosis
were
observed.
26
(50%)
patients
had
the
fever
for
more
than
5
days
and
17
patients
(32.7%)
with
fever
had
4
out
of
5
signs
of
Kawasaki,
and
the
other
35
patients
(3
/
67%)
were
considered
as
incomplete
or
atypical
Kawasaki.
Out
of
52
children
with
Kawasaki,
47
(90.4%)
responded
to
the
first
dose
of
intravenous
immunoglobulin
(group
A)
and
5
children
(9.6%)
did
not
respond
to
initial
treatment
(group
B).
35
(67.3%)
patients
were
male
and
17
(32.7%)
were
female.
30
male
patients
(85.7%)
and
all
female
patients
responded
to
the
first
dose
of
intravenous
immunoglobulin,
but
the
frequency
of
gender
distribution
in
the
two
groups
was
not
statistically
significant
(P
=
0.1).
Most
children
responded
to
the
first
dose
of
intravenous
immunoglobulin
and
all
single-dose
resistant
children
were
in
the
age
of
12-60
months.
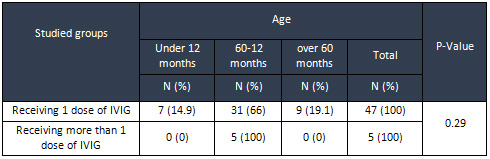
The
age
distribution
of
the
two
groups
was
not
statistically
significant
(P
=
0.
29)
(Table
1)
Table
1:
Frequency
Distribution
of
age
in
two
groups
The
mean
weight
of
children
receiving
the
intravenous
immunoglobulin
dose
was
between
15.5
±
5.2
Kg
and
the
mean
weight
of
children
resistant
to
treatment
was
14.2
±
1.48
kg
and
based
on
t-test
the
difference
was
not
statistically
significant
(P
=
0/59)
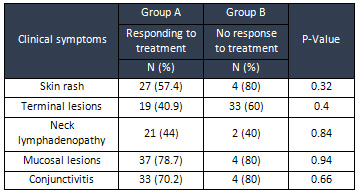
Differences
in
the
distribution
of
oral
lesions,
conjunctivitis,
neck
lymphadenopathy,
edema
and
cutaneous
rash
were
not
statistically
significant.
(Table
2)
Table
2:
Frequency
distribution
of
clinical
symptoms
on
admission
in
two
studied
groups
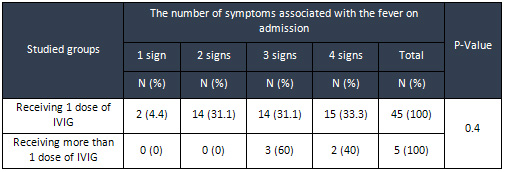
33.3%
of
the
respondents
to
the
initial
treatment
with
IV
immunoglobulin
had
fever
along
with
4
other
disease
signs,
while
most
of
the
resistant
children
to
the
treatment
had
3symptoms,
but
according
to
Fisher’s
exact
test,
the
difference
in
the
number
of
symptoms
associated
with
fever
on
admission
between
the
two
groups
was
not
statistically
significant
(P
=
0.04)
(Table
3)
Table
3:
Frequency
distribution
of
the
number
of
symptoms
associated
with
fever
on
admission
in
studied
groups
Click
here
for
Table
4:
The
Comparison
of
mean
and
standard
deviation
of
laboratory
indices
in
the
two
groups
In
comparing
the
laboratory
indices
between
the
two
groups,
the
results
showed
that
there
was
no
significant
difference
between
the
mean
values
of
hemoglobin
and
hematocrit
percentage,
liver
enzymes,
the
number
of
leukocytes,
the
percentage
of
polymorphonuclears
and
lymphocytes
and
the
number
of
platelets
in
the
two
groups.
Nevertheless,
the
mean
values
of
ESR
(P
=
0.002)
and
CRP
(P
=
0.011)
in
the
group
resistant
to
the
first
dose
of
intravenous
immunoglobulin
was
higher
than
that
of
the
responder
group,
and
this
difference
was
statistically
significant
based
on
t-test.
Also,
the
difference
in
serum
sodium
levels
was
statistically
significant
on
the
basis
of
t-test
between
the
two
groups
(p
=
0.017)
(Table
4
-
left).
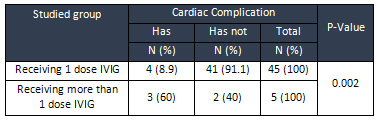
In
four
responder
patients
(8.9%)
to
the
first
dose
of
intravenous
immunoglobulin
a
heart
disease
was
recorded;
this
rate
was
60%
in
children
who
were
resistant
to
treatment
and
this
difference
was
statistically
significant
based
on
t-test
(P
=
0/002)
(Table
5).
Table
5:
Frequency
Distribution
of
cardiac
complication
possibility
in
the
studied
groups
| DISCUSSION
AND
CONCLUSION
|
Kawasaki
disease
is
an
engaging
vasculitis
in
multiple
organs,
of
unknown
etiology
and
the
main
cause
of
acquired
heart
disease
in
developed
countries
(9).
Diagnosis
is
based
on
fever
for
more
than
5
days
and
at
least
4
out
of
the
five
main
symptoms
of
the
disease
such
as
cutaneous
rash,
non-puerperal
bilateral
conjunctivitis,
neck
lymphadenopathy,
oral
mucosal
lesions,
and
edema
or
scaling
of
the
organs
(8).
Some
patients
may
not
have
the
necessary
diagnostic
criteria
from
the
beginning,
which
are
recognized
as
atypical
or,
preferably,
incomplete
Kawasaki,
since
many
patients’
symptoms
will
appear
in
the
course
of
the
disease
or
echocardiographic
findings
confirm
the
disease
(3).
Incomplete
types
are
more
common
in
children
of
lesser
age,
and
their
timely
identification
and
treatment
to
prevent
cardiac
problems
are
essential.
Distinct
differential
diagnoses
include
streptococcal
infections,
measles,
rheumatoid
arthritis,
and
drug
reactions
(16).
Prescribing
of
an
intravenous
immunoglobulin
dose
(2
g
/
kg)
plus
oral
aspirin
leads
to
improved
symptoms
and
signs
of
disease
and
reduces
the
risk
of
cardiac
problems
from
20%
to
about
5%
(17).
Unfortunately,
about
10-20%
of
patients
do
not
respond
to
classical
treatment
and
need
recurrence
doses
or
other
treatments
(18).
These
patients
are
classified
as
resistant
to
intravenous
immunoglobulins.
These
patients
are
at
greater
risk
for
heart
problems
(19).
Identifying
predictive
factors
for
treatment-resistant
types,
considering
other
treatments
or
the
probability
of
problems
occurrence
will
allow
them
to
be
treated
in
a
timely
manner.
Therefore,
this
study
was
conducted
to
identify
these
factors.
In
this
study,
demographic
factors
including
age,
sex,
weight,
and
laboratory
and
clinical
symptoms
were
compared
in
the
two
groups
of
responders
to
the
first
dose
of
intravenous
immunoglobulin
(A)
and
non-responders
to
the
first
dose
of
intravenous
immunoglobulin
(B).
A
total
of
52
patients
were
diagnosed
with
Kawasaki’s
final
diagnosis.
47
(90.4%)
patients
responded
to
intravenous
immunoglobulin
(group
A),
and
5
patients
(9.6%)
remained
febrile
48
hours
after
receiving
treatment
and
were
classified
as
resistant
(group
B).
There
was
no
significant
difference
in
age,
sex,
and
weight
between
the
two
groups
A
and
B.
In
a
study
conducted
by
Sungho
et
al.,
51
patients
were
evaluated,
33
of
whom
responded
to
treatment
with
IVIG
(64.7%)
and
13
patients
(35.3%)
classified
in
the
treatment-resistant
group.
The
percentage
of
people
resistant
to
treatment
in
this
study
was
higher
than
our
study.
In
the
study
of
Sungho
et
al.,
there
was
no
significant
difference
between
the
two
groups
in
terms
of
gender
and
age
(10).
In
another
study
conducted
by
Young
and
colleagues
on
82
Kawasaki
patients,
16
(19.5%)
patients
were
resistant
to
treatment,
and
here
as
well
there
was
no
difference
in
terms
of
sex,
age
and
weight
between
the
responders
and
those
who
were
resistant
to
treatment
(9).
In
our
study,
due
to
the
low
number
of
patients,
especially
the
cases
resistant
to
treatment
comparing
to
Sungho
et
al.
and
Tremoulet
et
al.
were
lack
of
high
sensitivity
concerning
other
studies
(10,
11).
In
our
study,
there
was
no
significant
difference
in
the
amount
of
hemoglobin,
hematocrit,
liver
transaminases,
and
the
number
of
leukocytes
and
neutrophils
and
lymphocytes
in
the
liver.
In
the
study
of
Sundel
et
al.
and
Wallace
et
al.,
there
was
no
difference
in
the
demographic
characteristics
and
clinical
manifestations
in
the
two
groups
while
hospitalized
(20,
23).
In
the
Sungho
study,
laboratory
tests
were
administered
on
two
groups
and
compared
to
the
time
of
admission
and
after
IVIG.
Hemoglobin,
hematocrit,
albumin,
ESR,
and
the
number
of
blood
leukocytes
were
not
significantly
different
between
the
two
groups,
while
the
percentage
of
polymorphonuclears
(PMNs)
and
bilirubin
and
AST
in
the
resistant
group
were
significantly
higher,
and
albumin
and
platelet
count
in
the
resistant
group
were
significantly
low.
After
prescription
of
IVIG,
hemoglobin
and
hematocrit
values,
total
protein
and
albumin
were
lower
in
the
resistant
group,
and
the
number
of
leukocytes,
the
percentage
of
PMNs,
ESR
and
total
and
direct
bilirubin
in
the
resistant
group
were
higher
and
there
was
not
a
significant
difference
in
the
levels
of
transaminases
and
CRP
(10).
In
our
study,
the
mean
values
of
hemoglobin,
hematocrit,
liver
transaminases,
the
number
of
leukocytes
and
platelets,
and
the
percentage
of
poly-Moreno
cells
and
blood
lymphocytes
were
not
significantly
different
in
the
two
groups.
Though,
the
mean
values
of
ESR
(P
=
0.002)
and
CRP
(P
=
0.017)
were
significantly
higher
in
the
treatment-resistant
group.
Correspondingly,
mean
serum
sodium
was
lower
in
the
treatment-resistant
group
(P
=
0.017).
Serum
albumin
and
bilirubin
value
were
not
included
in
our
study
due
to
the
lack
of
record
values
in
the
files.
Also
in
our
study,
laboratory
tests
were
considered
when
the
patients
were
hospitalized
since
none
of
the
patients
had
not
undergone
laboratory
tests
again
after
receiving
IVIG.
In
the
study
of
Uehara
et
al.,
the
relationship
between
high
hepatic
transaminases
and
the
incidence
of
cardiac
problems
in
patients
with
Kawasaki
has
been
described
(22).
In
the
study
of
Sungho
et
al.,
the
high
levels
of
transaminases
were
associated
with
resistant
varieties
and
Kawasaki
referral,
but
in
our
study,
there
was
no
relationship
between
the
level
of
liver
transaminases
and
treatment
resistance.
Tetsuya
et
al.
have
reported
high
levels
of
bilirubin,
transaminases
of
the
liver,
and
CRP
as
independent
predictors
of
resistance
to
IVIG
(25).
In
our
study,
there
was
a
correlation
between
high
CRP
level
and
treatment
resistance.
Bilirubin
values
were
not
recorded
in
the
files
and
liver
transaminases
were
not
included
in
predictor
factors.
In
our
study,
the
percentage
of
cardiac
problems
in
the
treatment-resistant
group
was
significantly
higher
than
the
response
group
(p
=
0.002)
(Table
5).
In
a
study
conducted
by
Uehara
et
al.,
the
incidence
of
cardiac
problems
was
significantly
higher
in
the
IVIG-resistant
group.
In
this
study,
male
gender,
the
onset
of
treatment
before
the
fifth
day
of
the
disease
and
recurrence
of
the
disease
were
also
known
as
factors
related
to
resistance
to
IVIG
(26).
Although
in
the
study
of
Taraguchi
et
al.,
there
was
no
significant
difference
in
the
incidence
of
cardiac
problems
in
patients
who
were
resistant
to
primary
treatment
with
IVIG
and
prednisolone
treatment
(24).
In
sum,
in
a
large
number
of
studies,
some
of
the
demographic
and
laboratory
factors
such
as
low
age,
the
onset
of
treatment
before
the
fifth
day
of
the
disease
(which
may
indicate
the
severity
of
the
disease),
low
platelet
levels,
sodium
and
albumin
levels,
raised
liver
transaminases,
and
Neutrophils
were
also
associated
with
resistance
to
treatment
and
the
incidence
of
cardiac
problems
(25).
Further
studies
are
needed
to
define
the
diagnostic
criteria
for
incomplete
Kawasaki
type
to
initiate
timely
treatment
(25).
Currently,
the
recommended
dose
for
Kawasaki
disease
is
high
doses
of
intravenous
immunoglobulin
and
aspirin,
but
in
the
case
of
primary
resistance
cases,
a
second
dose
of
intravenous
immunoglobulin
is
also
prescribed.
Considering
the
predictive
factors
for
treatment
resistance,
the
second
dose
of
intravenous
immunoglobulin
may
be
avoided
due
to
its
high
cost
and
other
adjunctive
treatments.
Sonoda
et
al.
have
considered
plasma
replacement
with
infliximab
prescription
as
effective
factors
in
reducing
the
symptoms
of
patients
who
have
been
resistant
to
the
second
dose
of
intravenous
administration
of
IVIG
(28).
In
our
study,
high
levels
of
ESR
and
CRP
and
low
sodium
levels
were
recognized
as
predictive
factors
for
treatment
resistance.
Among
the
limitations
of
this
study,
we
can
point
out
the
inadequacies
of
the
records,
which
led
to
the
elimination
of
a
number
of
patients
from
the
study
and
consequently
the
small
size
of
samples.
These
deficiencies
included
the
incompleteness
of
the
cases’
summary,
the
miscarriage
to
record
the
results
of
experiments,
the
absence
of
infection
course,
and
the
lack
of
patients’
following
up
in
terms
of
echocardiographic
findings.
Acknowledgments
We
hereby
appreciate
the
sincere
help
of
the
hardworking
staff
of
Shahid
Madani
Hospital
in
Khorramabad
for
helping
us
in
conducting
the
present
study.
1.
Kawasaki
T.
Acute
Febrile
mucocutaneous
syndrome
with
lymphoid
involvement
with
specific
desquamation
of
the
fingers
and
toes
in
children.
Arerugi;
1976:
16:
178.
2.
Danjani
AS.
Tanbert
KA.
Gerber
MA,
Shulman
ST,
Ferrieri
P,
Freed
M,
et
al.
Diagnosis
and
therapy
of
Kawasaki
disease
in
children.
Circulation.
1993;
87:
1776-1780.
3.
Klieman,
Stanton,
ST.
Geme,
Schore,
Behrman.
Nelson
text
book
of
ped.
19th
edition.
2011:
862-867.
4.
Falcini
F,
Ozen
S,
Magni-manasoni
S,
Candelli
M,
Racci
L,
Martini
G,
et
al.
Incomplete
and
atypical
kawaka
disease.
Clinical
exp
Rheumatol
2012
Sep-oct;
30(S):
799-800.
5.
Manlhioe
C,
Christie
E,
Mccrindle
BW,
Rosenburg
H,
Chanal
N,
Yeung
RSM.
Complete
and
incomplete
Kawasaki
disease:
two
sides
of
the
same
coin.
European
journal
of
pediatrics.
2012;
25
(3):
83-7.
6.
Nader
M
Osman.
Recurrent
Kawasaki
disease
resistance
to
initial
treatment
with
intravenous
Immunoglobulin.
Sudanese
journal
of
pediatrics.
2012;
12:
65-9.
7.
Kato
H,
Sugimura
T,
Akagi
T,
Sato
N,
Hashiro
K,
Maeno
Y,
et
al.
Long
term
Consequence
of
Kawasaki
disease.
Circulation.
1996;
94:
1379-85.
8.
Dasani
AS,
Tauberi
KA,
Gerber
MA,
Shulman
ST,
Ferreri
P,
Freed
M,
et
al.
Diagnosis
and
therapy
of
Kawasaki
disease
in
children.
Circulation.
1993;
87:
1776-80.
9.
Young
JHM,
Huwn
KF,
Chan
LTW.
Predictors
of
intravenous
immunoglobulin
resistance
in
Chinese
children
with
Kawasaki
disease.Hk
J
Pediatrics
.
2013;
18:
204-9.
10.
Sungho
C,
Minjeong
Y,
Yangjoo
A,
Miyoung
H,
Kinng-Lim
Y.
Risk
factors
for
failure
of
initial
immunoglobulin
treatment
in
Kawasaki
disease.
J
Korean
Med
Sci.
2008;
23:
718-22.
11.
Tremoulet
AH,
Best
BM,
Song
S,
Corinaldesi
E,
Eichofield
JR,
et
al.
Resistance
to
intravenous
immunoglobulin
in
children
with
Kawasaki
disease.
J
Pediatr.
2008;
153:
117-210.
12.
Fukuda
S,
Ito
S,
Sakai
H,
Kato
H,
Abe
J,
Ito
R,
et
al.
Late
development
of
coronary
artery
abnormalities
could
be
associated
with
persistence
non-fever
in
Kawasaki
disease.
Ped
Rheumatol
on
line
J.
2013;
11
(1):
2013
JW
31.
13.
Sleeper
LA,
Minich
LLA,
Mccrindle
BM,
Li
JS,
Mason
W,
Colon
SD,
et
al.
Evaluation
of
Kawasaki
disease
risk-scoring
systems
of
IVIG
resistance.
The
journal
of
pediatrics.
2011;
158:
831-50.
14.
Kuo
Hc,
Liang
CD,
Wang
CL,
YU
HR,
Hawang
KP,
Yanj
KD.
Serum
albumin
level
predict
initial
IVIG
treatment
failure
in
Kawasaki
disease.
Acta
paediatrica.
2012;
99
(10):
157-83.
15.
Kobayashi
T,
Imone
Y,
Takeuchi
K,
Okada
Y,
Tamura
K,
Tomomaso
T,
et
al.
Prediction
of
intravenous
immunoglobulin
unresponsiveness
in
patient
with
Kawasaki
disease.
Circulation.
2006;
113
(22):
2606-12.
16.
Davis
S,
Sutton
N,
Blockstock
S,
gommley
S,
Hoggart
CJ,
Levin
M,
et
al.
Predicting
IVIG
resistance
in
VK
Kawasaki
disease.
Archs
Dis
child,
published
online
10
Feb
2015.
17.
Burns
JC,
Wiggins
JW
Jr
,
Toews
WH,
Newburger
JW,
Young
DJ,
Wilson
H,
et
al.
Clinical
Spectrum
of
Kawasaki
disease
in
infants
younger
than
6
month
of
age.
J
Pediatr.
1986;
109:
759-63.
18.
Rowley
AH,
Gonzalez
CF,
Gidding
SS,
Duffy
CE,
Shulmon
ST.
Incomplete
Kawasaki
disease
with
coronary
artery
involvement.
J
Pediatr.
1987;
110:
409-13.
19.
Newburger
JW,
Takahashi
M,
Gerber
MA,
Gewts
MH,
Tani
LY,
Burns
JC.
Diagnosis,
treatment
and
long-term
management
of
Kawasaki
disease.
a
statement
for
health
professionals
for
the
committee
on
Rheumatic
fever,
endocarditis
and
Kawasaki
disease,
council
on
cardiovascular
disease
in
the
young.
American
hHeart
Association,
Circulation.
2004;
110:
2747-71.
20.
Sundel
RP,
Burns
JC,
Baker
AS,
Newburger
JW.
Gamma
globulin
re-treatment
in
Kawasaki
disease.
J
Pediatr.
1993;
123:
657-9.
21.
Koren
G,
Lavi
S,
Rose
V,
Rowe
R.
Kawasaki
disease:
review
of
risk
factor
for
coronary
aneurysms.
J
Pediatr.1986;
108:
388-392.
22.
Uehara
R,
Yashiro
M,
Hayasaka
S,
Oki
I,
Namakara
Y,
Mutaishii
M,
et
al.
Serum
alanine
amino
transferase
concentration
in
patient
with
Kawasaki
disease.
Ped
Infect
dis
J.
2003
sep;
22
(9):
839-42.
23.
Wallace
CA,
French
JW,
Kahn
SJ,
Sherry
DD.
Initial
intravenous
gamma
globulin
treatment
failure
in
Kawasaki
disease.
Pediatr.
2000
b
Jun;
105
(6):
E
78.
24.
Taraguchi
M,
Ogino
H,
Yoshimura
K,
Taninchi
S,
Kini
M,
Okasaki
H,
et
al.
Steroid
pulse
therapy
for
children
with
immunoglobulin
therapy-resistant
Kawasaki
disease.
Pediate
cardial.
2013
Apr;
34
(4):
959-63.
25.
Tetsuya
S,
Shunji
K,
Kouji
M,
Takushira
Y,
Inchiro
M,
Kazunori
M,
et
al.
Prediction
of
non-responsiveness
to
standard
high
dose
gamma-globulin
therapy
in
patients
with
acute
Kawasaki
disease
before
starting
initial
treatment.
26.
Uehera
R,
Belay
ED,
Maddex
RA,
Nakamara
Y,
Yashiro
M,
Oki
l,
et
al.
Analysis
of
potential
risk
factors
associated
with
nonresponse
to
initial
intravenous
immunoglobulin
treatment
among
Kawasaki
disease
patient
in
Japan.
Pediatr
infect
Dis.
2008
Feb;
27
(2):
155-60.
27.
Navaifar
MR,
Rezai
MS.
Intravenous
immunoglobulin
resistant
Kawasaki
disease.
J
Pediatr
Rev.
2013;
1
(1):
51-60.
28.
Sonoda
K,
Mori
M,
Hososaki
T,
Yokota
S.
Infliximab
plus
plasma
exchange
rescue
therapy
in
Kawasaki
disease.
J
pediatr.
2014;
164
(5):
1128.

