Accurate
estimates
of
virus
mutation
rates
are
important
to
understand
the
evolution
of
the
viruses
and
to
combat
them.
However,
methods
of
estimation
are
varied
and
often
complex.
The
mutation
rate
is
a
critical
parameter
for
understanding
viral
evolution
and
has
important
practical
implications.
For
example,
the
estimate
of
the
mutation
rate
of
HIV-1
demonstrated
that
any
single
mutation
conferring
drug
resistance
should
occur
within
a
single
day
and
that
simultaneous
treatment
with
multiple
drugs
was
therefore
necessary.
(1)
The
viral
mutation
rate
also
plays
a
role
in
the
assessment
of
possible
vaccination
strategies
and
it
has
been
shown
to
influence
the
stability
of
live
attenuated
polio
vaccines.
At
both
the
epidemiological
and
evolutionary
levels,
the
mutation
rate
is
one
of
the
factors
that
can
determine
the
risk
of
emergent
infectious
disease,
i.e.,
pathogens
crossing
the
species
barrier.
Slight
changes
of
the
mutation
rate
can
also
determine
whether
or
not
some
virus
infections
are
cleared
by
the
host
immune
system
and
can
produce
dramatic
differences
in
viral
fitness
and
virulence,
clearly
stressing
the
need
to
have
accurate
estimates.
(1)
Future
mutation
rate
studies
should
fulfil
the
following
criteria:
-
the
number
of
cell
infection
cycles
should
be
as
low
as
possible,
-
the
mutational
target
should
be
large,
-
mutations
should
be
neutral
or
lethal
or
a
correction
should
be
made
for
selection
bias.
Adhering
to
these
criteria
will
help
us
to
obtain
a
clearer
picture
of
virus
mutation
patterns.
(1)
There
have
been
many
laboratory-based
investigations
since
the
emergence
of
the
new
coronaviruses
in
2012,
but
most
of
the
parameters
required
for
establishing
scientifically
the
control
measures
that
will
protect
against
them
have
yet
to
be
determined.
Equally,
the
global
distribution
of
the
viruses
in
their
animal
reservoir
has
yet
to
be
established.
The
approach
to
monitoring
of
virus
mutation
is
to
highlight
particular
questions
that
need
to
be
answered
for
the
purposes
of
preventing
or
treating
these
infections
and
diseases.
Tables
1-3,
provide
a
summary
of
data
and
investigations
required
for
control
or
mitigation
of
virus
spread.
Table
1:
Information
required
from
investigations
for
control
or
mitigation
of
a
novel
respiratory
virus
affecting
humans
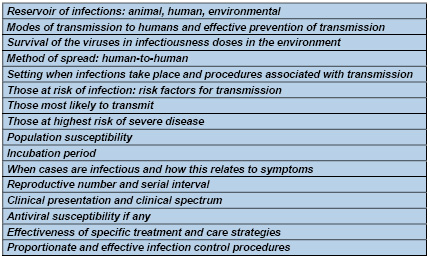
Table
2:
What
parameters
are
involved
in
virus
spread?
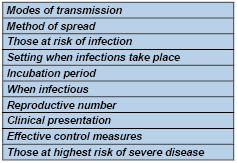
Table
3:
Specific
public
health
questions
regarding
novel
corona
viruses
that
need
to
be
answered
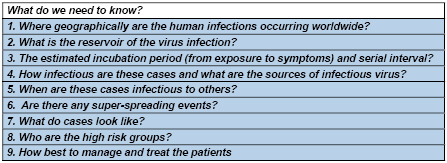
Dealing
with
virus
outbreaks
Viruses
cannot
exist
on
their
own
and
for
survival
they
need
to
spread
to
another
host.
This
is
because
the
original
host
may
either
die
or
eliminate
the
infection.
Some
important
routes
of
viral
transfer
include:

| GLOBAL
AND
REGIONAL
VIRUS
UDATES
|
MERS
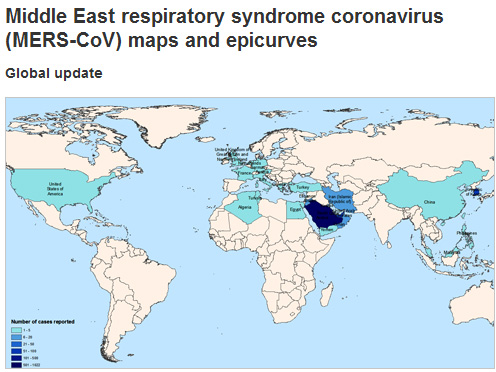
Reproduced
with
permission
from:
World
Health
Organization
Coronaviruses
are
a
large
and
diverse
family
of
viruses
that
include
viruses
that
are
known
to
cause
illness
in
humans.Middle
East
Respiratory
Syndrome
coronavirus
(MERS-CoV)
has
never
previously
been
detected
in
humans
or
animals
but
appears
most
closely
related
to
coronaviruses
previously
found
in
bats.
It
is
genetically
distinct
from
the
SARS
coronavirus,
and
appears
to
behave
differently.
The
World
Health
Organization
(WHO)
first
reported
cases
of
Middle
East
Respiratory
syndrome
(MERS)
coronavirus
on
23
September
2012.
While
Saudi
Arabia
has
still
recorded
the
highest
number
of
MERS
deaths,
(over
400)
the
outbreak
continues
in
South
Korea
with
33
deaths
and
183
cases
to
mid
June
2015.
All
cases
have
lived
in
or
travelled
to
the
Middle
East,
or
have
had
close
contact
with
people
who
acquired
the
infection
in
the
Middle
East.
See
map
above
for
current
detail
on
global
and
regional
numbers
of
cases
reported.
MERS
Symptoms
•
Most
people
become
unwell
quickly,
with
fever,
cough,
shortness
of
breath,
leading
to
pneumonia.
•
Other
symptoms
include
muscle
pain,
diarrhoea,
vomiting
and
nausea.
•
There
have
also
been
people
with
mild
symptoms
or
no
symptoms
at
all.
These
people
had
close
contact
with
others
who
were
seriously
ill.
How
MERS
spreads
•
It
appears
to
spread
from
an
infected
person
to
another
person
in
close
contact.
The
virus
does
not
appear
to
spread
easily
from
person-to-person
and
appears
to
spread
only
from
people
who
are
sick.
•
Some
people
in
the
Middle
East
appear
to
have
caught
the
disease
from
infected
camels
and
bats.
How
this
occurred
is
not
well
understood.
People
with
underlying
illnesses
that
make
them
more
vulnerable
to
respiratory
disease
may
be
at
a
higher
risk.
How
it
is
diagnosed
A
laboratory
test
on
fluid
collected
from
the
back
of
the
throat
or
the
lungs
can
diagnose
MERS-CoV.
How
it
is
treated
There
is
no
vaccine
for
MERS-CoV
but
early
and
careful
medical
care
can
save
lives.
Key
facts
•
Middle
East
respiratory
syndrome
(MERS)
is
a
viral
respiratory
disease
caused
by
a
novel
coronavirus
(MERS-CoV)
that
was
first
identified
in
Saudi
Arabia
in
2012.
•
Coronaviruses
are
a
large
family
of
viruses
that
can
cause
diseases
ranging
from
the
common
cold
to
Severe
Acute
Respiratory
Syndrome
(SARS).
•
Typical
MERS
symptoms
include
fever,
cough
and
shortness
of
breath.
Pneumonia
is
common,
but
not
always
present.
Gastrointestinal
symptoms,
including
diarrhoea,
have
also
been
reported.
•
Approximately
36%
of
reported
patients
with
MERS
have
died.
•
Although
the
majority
of
human
cases
of
MERS
have
been
attributed
to
human-to-human
infections,
camels
are
likely
to
be
a
major
reservoir
host
for
MERS-CoV
and
an
animal
source
of
MERS
infection
in
humans.
However,
the
exact
role
of
camels
in
transmission
of
the
virus
and
the
exact
route(s)
of
transmission
are
unknown.
•
The
virus
does
not
seem
to
pass
easily
from
person
to
person
unless
there
is
close
contact,
such
as
occurs
when
providing
unprotected
care
to
a
patient.
Between
1
and
4
June
2015,
the
National
IHR
Focal
Point
for
the
Kingdom
of
Saudi
Arabia
notified
WHO
of
5
additional
cases
of
Middle
East
respiratory
syndrome
coronavirus
(MERS-CoV)
infection,
including
1
death.
Contact
tracing
of
household
and
healthcare
contacts
is
ongoing
for
these
cases.
In
patients
with
suspected
pneumonia
or
pneumonitis
with
a
history
of
recent
residence
or
travel
(in
the
14
days
prior
to
symptom
onset)
in
the
Middle
East*,
or
close
contact
with
confirmed
or
probable
cases,
the
following
is
recommended:
1.
The
patient
should
be
placed
in
a
single
room
if
available
and
standard
and
transmission-based
precautions
implemented
(contact,
droplet
and
airborne),
including
the
use
of
personal
protective
equipment
(PPE).
2.
The
relevant
state/territory
public
health
unit/communicable
diseases
branch
must
be
notified
urgently
of
any
suspected
(and
probable
or
confirmed)
cases
in
order
to
discuss
patient
referral
and
coordinate
management
of
contacts.
Note:
Transiting
through
an
international
airport
(<24hours
duration,
remaining
within
the
airport)
in
the
Middle
East
is
not
considered
to
be
risk
factor
for
infection.
Are
GPs/FPs
at
risk
from
MERS-CoV?
Many
confirmed
cases
have
occurred
in
healthcare-associated
clusters,
and
there
have
been
a
large
number
of
cases
in
healthcare
workers,
but
mainly
in
hospital
settings
as
has
predominantly,
if
not
exclusively,
been
the
case
in
South
Korea.
The
particular
conditions
or
procedures
that
lead
to
transmission
in
hospital
are
not
well
known.
However,
lapses
in
infection
control
were
known
to
have
occurred
for
seven
healthcare
workers
who
acquired
the
infection
from
cases
in
Saudi
Arabia.
Patient
Pre-travel
advice,
travel
restrictions,
periods
of
peak
travel
The
WHO
does
not
currently
recommend
any
restrictions
to
travel
due
to
the
MERS-CoV
outbreak.
Travellers
should
be
aware
of
the
importance
of
personal
hygiene
including
frequent
hand
washing,
avoiding
close
contact
with
animals
and
with
people
who
are
suffering
from
acute
respiratory
infection,
and
should
be
advised
to
seek
medical
attention
as
soon
as
possible
if
they
feel
unwell.
They
should
also
follow
usual
food
hygiene
practices
for
travellers,
including
avoiding
drinking
raw
milk
or
eating
food
that
may
be
contaminated
with
animal
secretions
or
products
unless
they
are
properly
washed,
peeled
or
cooked.
What
are
the
recommended
isolation
and
PPE
recommendations
for
patients
in
hospital?
In
summary,
transmission-based
precautions
for
suspected,
probable
and
confirmed
cases
should
include:
•
Placement
of
confirmed
and
probable
cases
in
a
negative
pressure
room
if
available,
or
in
a
single
room
from
which
the
air
does
not
circulate
to
other
areas
•
Airborne
transmission
precautions,
including
routine
use
of
a
P2
respirator,
disposable
gown,
gloves,
and
eye
protection
when
entering
a
patient
care
area
•
Contact
precautions,
including
close
attention
to
hand
hygiene
•
If
transfer
of
the
confirmed
or
probable
case
outside
the
negative
pressure
room
is
necessary,
asking
the
patient
to
wear
a
surgical
face
mask
while
they
are
being
transferred
and
to
follow
respiratory
hygiene
and
cough
etiquette.
Ebola
Ebola
is
spread
through
contact
with
blood
or
other
body
fluids,
or
tissue
from
infected
people
or
animals.
The
known
strains
vary
dramatically
in
their
fatality
rates.
The
Bundibugyo
strain
fatality
rate
is
up
to
50
percent,
and
it
is
up
to
71
percent
for
the
Sudan
strain,
according
to
WHO.
Less
than
two
months
after
Liberia
was
declared
Ebola-free
by
the
World
Health
Organization,
the
virus
is
back
in
the
country.
Even
when
the
outbreak
diminished
in
Liberia,
neighboring
Guinea
and
Sierra
Leone
have
continued
to
see
20
to
27
cases
a
week
since
late
May
2015,
according
to
the
WHO.
There
have
been
more
than
11,000
total
deaths
from
the
outbreak
since
it
began
in
March
2014.
Ebola
Situation
Report
-
8
July
2015
There
were
30
confirmed
cases
of
Ebola
virus
disease
(EVD)
reported
in
the
week
to
5
July
2015:
18
in
Guinea,
3
in
Liberia,
and
9
in
Sierra
Leone.
Ebola
Situation
Report
-
1
July
2015
There
were
20
confirmed
cases
of
Ebola
virus
disease
(EVD)
reported
in
the
week
to
28
June,
the
same
as
the
previous
week.
Weekly
case
incidence
has
been
between
20
and
27
cases
for
5
consecutive
weeks.
In
Guinea,
12
cases
were
reported
from
3
prefectures:
Boke,
Conakry,
and
Forecariah.
Chikungunya
virus
While
not
fatal,
this
virus
can
have
a
chronic
disabling
effect
and
it
has
spread
rapidly
around
the
globe.
Chikungunya
is
ravaging
the
Caribbean,
having
affected
24
Caribbean
nations
and
possibly
more
than
850,000
people
worldwide,
including
185
Americans
(in
New
Jerseyans).
Chikungunya
virus
is
most
often
spread
to
people
by
Aedes
aegypti
and
Aedes
albopictus
mosquitoes.
These
are
the
same
mosquitoes
that
transmit
dengue
virus.
•
The
only
way
to
prevent
chikungunya
is
to
prevent
mosquito
bites,
such
as
by
using
repellant.
•
Several
vaccines
are
in
the
developmental
stage
but
none
are
in
the
licensing
stage.
•
Generally,
more
South
Jersey
counties
have
a
higher
risk
because
they
have
more
Asian
Tiger
Mosquitoes.
It
is
predicted
that
chikungunya
virus
will
spread
through
rest
of
the
globe
this
year
(2015).
•
Prior
to
2013,
chikungunya
virus
outbreaks
had
been
identified
in
countries
in
Africa,
Asia,
Europe,
and
the
Indian
and
Pacific
Oceans.
•
In
late
2013,
the
first
transmission
of
chikungunya
virus
in
the
Americas
was
identified
in
Caribbean
countries
and
territories.
Local
transmission
means
that
mosquitoes
in
the
area
have
been
infected
with
the
virus
and
are
spreading
it
to
people.
•
Since
then,
local
transmission
has
been
identified
in
44
countries
or
territories
throughout
the
Americas
with
more
than
1.2
million
suspected
cases
reported
to
the
Pan
American
Health
Organization
from
affected
areas.
Symptoms
•
Most
people
infected
with
chikungunya
virus
will
develop
some
symptoms.
•
Symptoms
usually
begin
3-7
days
after
being
bitten
by
an
infected
mosquito.
•
The
most
common
symptoms
are
fever
and
joint
pain.
•
Other
symptoms
may
include
headache,
muscle
pain,
joint
swelling,
or
rash.
•
Chikungunya
disease
does
not
often
result
in
death,
but
the
symptoms
can
be
severe
and
disabling.
•
Most
patients
feel
better
within
a
week.
In
some
people,
the
joint
pain
may
persist
for
months.
•
People
at
risk
for
more
severe
disease
include
newborns
infected
around
the
time
of
birth,
older
adults
(>65
years),
and
people
with
medical
conditions
such
as
high
blood
pressure,
diabetes,
or
heart
disease.
•
Once
a
person
has
been
infected,
he
or
she
is
likely
to
be
protected
from
future
infections.
SARS
Severe
acute
respiratory
syndrome.
No
outbreaks
since
May
2004
China
Avian
Flu
Avian
influenza
A
(H7N9)
is
a
subtype
of
influenza
viruses
that
have
been
detected
in
birds
in
the
past.
This
particular
A
(H7N9)
virus
had
not
previously
been
seen
in
either
animals
or
people
until
it
was
found
in
March
2013
in
China.
However,
since
then,
infections
in
both
humans
and
birds
have
been
observed.
The
disease
is
of
concern
because
most
patients
have
become
severely
ill.
Most
of
the
cases
of
human
infection
with
this
avian
H7N9
virus
have
reported
recent
exposure
to
live
poultry
or
potentially
contaminated
environments,
especially
markets
where
live
birds
have
been
sold.
This
virus
does
not
appear
to
transmit
easily
from
person
to
person,
and
sustained
human-to-human
transmission
has
not
been
reported.
WHO
risk
assessment
of
human
infection
with
avian
influenza
A
(H7N9)
virus
On
23
February
2015
WHO
conducted
a
risk
assessment
in
accordance
with
the
WHO
recommendations
for
rapid
risk
assessment
of
acute
public
health
events
the
summary
can
be
found
below.
Risk
assessment
This
23
February
2015
risk
assessment
was
conducted
in
accordance
with
WHO's
published
recommendations
for
rapid
risk
assessment
of
acute
public
health
events
and
will
be
updated
as
more
information
becomes
available.
Overall,
the
public
health
risk
from
avian
influenza
A(H7N9)
virus
has
not
changed
since
the
assessment
published
on
2
October
20142.
What
is
the
likelihood
that
additional
human
cases
of
infection
with
avian
influenza
A
(H7N9)
viruses
will
occur?
The
understanding
of
the
epidemiology
associated
with
this
virus,
including
the
main
reservoirs
of
the
virus
and
the
extent
of
its
geographic
spread
among
animals,
remains
limited.
However,
it
is
likely
that
most
human
cases
were
exposed
to
the
H7N9
virus
through
contact
with
infected
poultry
or
contaminated
environments,
including
markets
(official
or
illegal)
that
sell
live
poultry.
Changes
to
hygiene
practices
in
live
poultry
markets
have
been
implemented
in
many
provinces
and
municipalities.
Since
the
virus
source
has
not
been
identified
nor
controlled,
and
the
virus
continues
to
be
detected
in
animals
and
environments
in
China,
further
human
cases
are
expected
in
affected
and
possibly
neighbouring
areas.
What
is
the
risk
of
international
spread
of
avian
influenza
A
(H7N9)
viruses
by
travellers?
On
27
and
31
Jan
2015,
Canada
reported
2
cases
of
human
infection
with
avian
influenza
A
(H7N9)
in
travellers
returning
from
China.
These
travellers
had
mild
symptoms
and
only
reported
indirect
contact
with
poultry.
On
12
February
2014,
Malaysia
reported
one
human
case
with
avian
influenza
A
(H7N9)
virus
infection.
The
patient
was
a
Chinese
resident
who
travelled
to
Malaysia
while
sick,
and
was
most
likely
exposed
in
China.
No
further
cases
were
reported
in
Malaysia
linked
to
this
case.
It
is
possible
that
further
similar
cases
will
be
detected
in
other
countries
among
travellers
from
affected
areas,
although
community-level
spread
in
these
other
countries
is
unlikely.
Flu
viruses
During
a
typical
flu
season,
up
to
500,000
people
worldwide
will
die
from
the
illness,
according
to
WHO.
But
occasionally,
when
a
new
flu
strain
emerges,
a
pandemic
results
with
a
faster
spread
of
disease
and,
often,
higher
mortality
rates.
There
are
four
types
of
virus
that
cause
seasonal
flu
in
humans.
Every
year,
drug
developers
try
to
predict
which
strains
are
likely
to
dominate
in
the
next
flu
season
so
as
to
create
an
effective
flu
vaccine.
A
good
understanding
of
the
rate
and
pattern
of
virus
evolution
helps
these
predictions,
as
one
of
the
authors,
Dr.
Ian
Barr,
of
the
World
Health
Organization
(WHO)
Collaborating
Centre
for
Reference
and
Research
on
Influenza
in
Melbourne,
Australia,
explains:
"This
work
represents
another
piece
in
the
complex
puzzle
of
influenza
virus
circulation
and
human
infections
and
provides
insights
that
will
help
develop
better
influenza
vaccines
that
match
strains
circulating
in
the
community."
The
four
viruses
that
cause
seasonal
flu
in
humans
are:
influenza
A
viruses
H3N2
and
H1N1,
and
influenza
B
viruses
Yamagata
and
Victoria.
The
viruses
cause
similar
symptoms
-
for
instance
sudden
fever,
tiredness
and
weakness,
dry
cough,
headache,
chills,
muscle
aches,
sore
throat
-
and
they
evolve
in
similar
ways.
But
what
has
not
been
well
understood
is
their
different
patterns
of
spread
around
the
world
and
what
influences
them.
H1N1
and
B
viruses
persist
locally
between
epidemics.
Marburg
virus
Scientists
identified
Marburg
virus
in
1967,
when
small
outbreaks
occurred
among
lab
workers
in
Germany
who
were
exposed
to
infected
monkeys
imported
from
Uganda.
Marburg
virus
is
similar
to
Ebola
in
that
both
can
cause
hemorrhagic
fever,
meaning
that
infected
people
develop
high
fevers
and
bleeding
throughout
the
body
that
can
lead
to
shock,
organ
failure
and
death.
The
mortality
rate
in
the
first
outbreak
was
25
percent,
but
it
was
more
than
80
percent
in
the
1998-2000
outbreak
in
the
Democratic
Republic
of
Congo,
as
well
as
in
the
2005
outbreak
in
Angola,
according
to
the
World
Health
Organization
(WHO).
Rabies
Although
rabies
vaccines
for
pets,
which
were
introduced
in
the
1920s,
have
helped
make
the
disease
exceedingly
rare
in
the
developed
world,
this
condition
remains
a
serious
problem
in
India
and
parts
of
Africa.
It
destroys
the
brain,
but
there
is
a
vaccine
against
rabies,
and
we
have
antibodies
that
work
against
rabies,
so
if
someone
gets
bitten
by
a
rabid
animal
they
can
be
treated,
If
a
patient
doesn't
get
treatment,
there's
a
100
percent
possibility
they
will
die.
HIV
In
the
modern
world,
the
deadliest
virus
of
all
may
be
HIV.
It
is
still
the
biggest
killer.
An
estimated
36
million
people
have
died
from
HIV
since
the
disease
was
first
recognized
in
the
early
1980s.
Powerful
antiviral
drugs
have
made
it
possible
for
people
to
live
for
years
with
HIV.
But
the
disease
continues
to
devastate
many
low-
and
middle-income
countries,
where
95
percent
of
new
HIV
infections
occur.
Nearly
1
in
every
20
adults
in
Sub-Saharan
Africa
is
HIV-positive,
according
to
WHO.
Dengue
Dengue
virus
first
appeared
in
the
1950s
in
the
Philippines
and
Thailand,
and
has
since
spread
throughout
the
tropical
and
subtropical
regions
of
the
globe.
Up
to
40
percent
of
the
world's
population
now
lives
in
areas
where
dengue
is
endemic,
and
the
disease
-
with
the
mosquitoes
that
carry
it
-
is
likely
to
spread
farther
as
the
world
warms.
Dengue
sickens
50
to
100
million
people
a
year,
according
to
WHO.
Although
the
mortality
rate
for
dengue
fever
is
lower
than
some
other
viruses,
at
2.5
percent,
the
virus
can
cause
an
Ebola-like
disease
called
dengue
hemorrhagic
fever,
and
that
condition
has
a
mortality
rate
of
20
percent
if
left
untreated.
Rotavirus
Two
vaccines
are
now
available
to
protect
children
from
rotavirus,
the
leading
cause
of
severe
diarrheal
illness
among
babies
and
young
children.
The
virus
can
spread
rapidly,
through
what
researchers
call
the
fecal-oral
route
(meaning
that
small
particles
of
feces
end
up
being
consumed).
Although
children
in
the
developed
world
rarely
die
from
rotavirus
infection,
the
disease
is
a
killer
in
the
developing
world,
where
rehydration
treatments
are
not
widely
available.
The
WHO
estimates
that
worldwide,
453,000
children
younger
than
age
5
died
from
rotavirus
infection
in
2008.
But
countries
that
have
introduced
the
vaccine
have
reported
sharp
declines
in
rotavirus
hospitalizations
and
deaths.
The
severity
of
viral
outbreaks
will
largely
depend
on
the
local,
regional
and
global
response
to
them.
Early
vigilance
by
public
health
authorities
and
family
doctors
in
endemic
areas,
particularly,
are
the
greatest
preventive
measure
along
with
hygienic
practices
of
people,
especially
those
living
in
close
proximity
to
animal
or
bird
carriers
and
those
in
hospital
situations.
Global
measures
will
need
to
be
enacted
early
and
up
to
date
information
made
available
to
limit
spread
when
it
does
occur.
Ideally,
as
in
the
case
of
smallpox
which
was
declared
eradicated
in
1980
following
a
global
immunization
campaign
led
by
the
World
Health
Organization,
we
can
start
to
tackle
both
the
initial
outbreaks
and
the
spread
of
the
more
life
threatening
viruses.
This
takes
money
and
global
will.
(1)
Nicoll
A.
Short
communication.
Public
health
investigations
required
for
protecting
the
population
against
novel
coronaviruses
(2)
WHO
Disease
Outbreak
(3)
http://www.cdc.gov/chikungunya/symptoms/index.html
(4)
http://www.who.int/csr/don/en/
(5)
Rafael
Sanjuán,
Miguel
R.
Nebot,
Nicola
Chirico,
Louis
M.
Mansky
and
Robert
Belshaw
Viral
Mutation
Rates.
Journal
of
Virology.
July
2010

