|
Comparative Analysis
of Antimicrobial Peptides Gene Expression in
Susceptible/Resistant Mice Macrophages to Leishmania
major Infection
Hamid Daneshvar (1)
Iraj Sharifi (2,3)
Alireza Kyhani (3)
Amir Tavakoli Kareshk (2)
Arash Asadi (2)
(1) Department
of Immunology, School of Medicine, Kerman University
of Medical Sciences, Kerman, Iran
(2) Department of Parasitology and Mycology,
School of Medicine, Kerman University of Medical
Sciences, Kerman, Iran
(3) Leishmaniasis Research Center, Kerman University
of Medical Sciences, Kerman, Iran
Corresponding author:
Arash
Asadi
Department of Parasitology and Mycology,
School of Medicine,
Kerman University of Medical Sciences,
Kerman, Iran
Email: asadiarash209@yahoo.com
|
Abstract
Introduction and Objective:
BALB/c and C57BL/6 mouse strains represent
immunologically different responses to
Leishmania major infection. Antimicrobial
peptides (AMPs) for example, cathelicidins
and defensins, are unique compounds of
innate immunity system with multifunctional
effects against invasive pathogens. Nevertheless,
they have been less studied in parasitic
fields. The aim of the present study was
to evaluate the role of AMPs in susceptibility
or resistance to L.major infection.
Methodology:
Macrophages derived from peritoneal
cavity of BALB/c and C57BL/6 mouse strains
were exposed to the stationary phase of
L. major promastigotes for 3 hours, 24
hours and 7 days. Cell sediments and supernatants
from infected (test) and uninfected groups
(control) at 3 hours, 24 hours and on
7 days were used for the assessment of
infection severity, gene expression of
various mouse beta defensins (mBD), Cathelin-related
antimicrobial peptide (CRAMP), interleukin
(IL)-10, IL-12 and protein assay under
standard methods, respectively.
Findings:
Based on cytokine profiles evaluated in
BALB/c (IL-10, IL-12) and C57BL/6 derived
macrophages (IL-10, IL-12), the immunity
system was stimulated differently during
infection. The inter assay analysis revealed
that the test group of BALB/c derived
macrophages significantly expressed an
up-regulation of CRAMP, mBD1 genes and
their related proteins, when they are
challenged with L. major parasites. Nevertheless,
they also showed infection severity more
than those in other strains.
Conclusion: Due to higher expression
and release of AMPs by BALB/c derived
macrophages, the L. major infection ultimately
occurs in BALB/c mice. On the other hand,
the release of AMPs is important, but
cannot create an absolute protection against
leishmania infection.
Key words:
Antimicrobial Peptides, Cathelin-Related
Antimicrobial Peptide, Murine b-Defensin,
Leishmania major, Cytokin
|
Leishmaniasis is an arthropod-borne disease
created by intracellular protozoan parasites
of the Leishmania genus (Vega-López,
2012). It is a very important health problem
of the recent century in 98 countries and territories
(Alvar et al., 2012). The endemic areas of human
infections are present mainly in tropics, subtropics,
southern Europe and western Asia (Ashford, 1997,
Desjeux, 1996). Depending on species and host
immunity, several complications such as cutaneous
(CL), muccocutaeous (MCL) and visceral leishmaniasis
(VL) have been recognized (Herwaldt, 1999).
L. major is an ethological agent of CL in different
countries such as Iran (Le Blancq et al., 1986,
Azizi et al., 2016). Infection is caused, when
a female sand-fly inoculates the metacyclic
phase of L. major promastigotes into the dermis
of a suitable vertebrate host (Dostálová
and Volf, 2012). The parasites are engulfed
by macrophages for further development (Handman
and Bullen, 2002). More interestingly, macrophages
involve in both development and killing of parasites.
Previous studies have shown that susceptible
(BALB/c) and resistant (C57BL/6) mouse strains
represent different immune responses to L. major
infection (Hejazi et al., 2012, Lazarski et
al., 2013, Park et al., 2000). Based on the
important role of macrophages, it is necessary
to know if new mechanisms such as antimicrobial
peptides (AMPs) are employed by them following
L. major infection. Historically, the first
AMPs was isolated from a soil Bacillus strain
and named gramicidin (Dubos, 1939). More than
5,000 AMPs have been identified so far (Zhao
et al., 2013). Cathilicidins and defensins are
two main groups of AMPs (Ganz, 2003, Lehrer
and Ganz, 2002a). Cathelin-related antimicrobial
peptide (CRAMP) is the only cathelicidin found
in mouse strains and expressed by different
kinds of cells or tissues, while a variety of
mouse beta defensins (mBD) have been identified
(Dorschner et al., 2003, Nizet and Gallo, 2003,
Bardan et al., 2004). They kill or inhibit invasion
pathogens through direct effects or modulation
of inflammatory responses (Deng et al., 2016,
Hemshekhar et al., 2016, Chromek et al., 2012,
Kovach et al., 2012). Despite being remarkable
cases of CL, little study is found about AMPs
role in Leishmania infections. The present study
aimed to show whether AMPs can affect susceptibility
or resistance to L. major infection.
Ethics approval and consent to participate:
To work on animals, we obtained permission
number ir.kmu.rec.1394.208 from the ethical
board of Kerman University of Medical Science
(Kerman, Iran).
Parasite: L. major (strain MRHO/IR/75/ER,
Iranian type collection) was purchased from
Razi Institute (Karj, Iran) and cultured in
50 ml flask containing RPMI 1640 enriched with
10% heated-inactivated fetal bovine serum (HFBS)
and 1% penicillin/streptomycin (pen/strep) antibiotics.
Macrophages Isolation: Macrophages were
isolated from BALB/c (n=5) and C57BL/6 (n=5)
mouse strains from peritoneal cavity like the
previous study (Ray and Dittel, 2010), and cultured
in Dulbecco’s Modified Eagle’s Medium
(DMEM) enriched with 10% HFBS and 1% pen/strep
antibiotics. The cells were incubated at 37oC
in 5% CO2 in humid conditions. For the experiments,
the macrophages derived from each strain were
separately placed in two sub groups: non-infected
(control) and challenged by L. major (test).
Co-incubation of Macrophages with parasite:
Macrophage (106 /well) from both strains were
separately transferred into 24-wells cell culture
plates. Each cell culture plate was designated
for one defined time and selected group (5 well
for test groups and 5 well for controls). The
cells were incubated at 37oC for 6 hours and
non adherent cells removed. The stationary phase
of L. major promastigotes (10:1) was added only
to test groups and incubated at 37oC for 3 hours.
The free promastigotes were removed and the
cells were incubated at 37oC for an additional
24 hours and 7 days.
Microscopic observation: To measure
parasite burden, the test groups were stained
using routine Giemsa staining method 3 hours
post infection according to a previous study
(Faber et al., 2003). Parasite burden (number
of parasites per macrophage) and infection rate
(% infected macrophages) were obtained by counting
the intracellular amastigotes using a light
microscope (Nikon, Japan).
Quantitative Real-Time PCR: For analysis
of cytokines and AMPs gene expression, whole
macrophages from test and control groups were
separately harvested at 24 hours post infection
with parasites. Total RNA was extracted using
RNA Purification kit (Jena Bioscience, Germany)
and quantified by a NanoDrop 2000 spectrophotometer
(Thermo Scientific, Wilmington, DE). Three mg
was transcribed to complementary DNA (cDNA)
using AccuPower®RT PreMix random hexaprimer
(Bioneer, Korea). Briefly, 3 µg of RNA
was adjusted in 20 µl DEPCI-DW and totally
added to each lyophilized tube. Thermal profile
was performed as following: 12 cycles (20ºC
for 30 seconds, 42ºC for 4 minutes, 55ºC
for 30 seconds) and 95ºC for 5 minutes.
Quantitative Real-time PCR was utilized using
a Rotor GENE Q (Qiagen, Germany). RPII was used
to amplify house-keeping cDNA. Other primers
were applied to amplify desirable amount of
cDNA (Table 1). Briefly, The 15l of each reaction
mixture (1ml cDNA, 7 ml SYBR Green, 5ml DW,
1mL primer forward 2.5 Pmol, 1mL primer reverse
2.5 Pmol) was prepared using SYBR Premix EX
Taq2 Master Mix (Takara, Japan). Thermal profile
was performed as following: 95°C for 1 minute,
40 cycles (94°C for 15 seconds, 58°C
for 30 seconds, 72°C for 20 seconds).
ELISA for protein assay: Macrophages
were exposed with the stationary phase of L.
major promastigotes and kept for 7 days. Due
the fact that CRAMP is secreted into cell culture
media by macrophages, supernatants were collected
for CRAMP assessment by enzyme-linked immunosorbent
assay (ELISA). CRAMP assay was accessed using
direct ELISA. In this method, the concentration
of CRAMP is equal with the absorbance of optical
density (OD), and mean of the final OD was calculated
as final results. Briefly, a 96-well plate was
coated with 5 µg of each supernatant in
50 µl of 0.1M carbonate buffer PH 9.6
and incubated at 4°C FOR 18h. After 3 washes
with 300µl of PBS, pH 7.2, 0.1% Tween-20,
the plate was then blocked with 100 µl
of blocking buffer (PBS, FBS 10%) and incubated
at 37°C for 1 hour. Following 3 washes,
100 µl of 1:200 (in PBS, Ph 7.2, 0.1%
Tween-20) of horse radish peroxidase conjugated
CRAMP antibody (Santacruz, California) was added
and incubated for 1 hour at 37º C and washed
3 times at the end of incubation. The plate
was incubated with 100 µl of substrate
solution for 30 minutes. In the final step,
50µl of stop solution was added and optical
density (OD492) detected using ELx800 micro
plate reader (BioTek, USA).
Parasite
burden:
Initially,
we
assessed
infectivity
rate
and
parasite
burden
of
test
groups
3
hours
post
co-incubation.
C57BL/6
derived
macrophages
had
a
significant
reduction
of
infection
rate
(24.5±0.31)
as
compared
to
(45±0.73)
for
BALB/C
derived
macrophages
(Figure
1.
A).
In
the
next
step,
we
characterized
the
parasite
burden
by
counting
the
number
of
intracellular
amastigotes
per
macrophage.
We
saw
a
significant
reduction
of
parasite
burden
(2.78±0.10
parasite/macrophage)
for
C57BL/6
derived
macrophages
as
compared
to
(8.68±0.22)
for
other
strain
(Figure
1.
B).
Antimicrobial
peptides
expression:
Real-time
PCR
was
applied
to
measure
the
mRNA
of
defined
AMPs
following
L.
major
infection.
The
results
were
analyzed
under
CT
method.
In
BALB/c
derived
macrophages,
the
test
groups
expressed
all
aforementioned
genes
more
than
their
controls
except
mBD2,
but
significantly
up-regulation
was
documented
only
for
CRAMP
and
mBD1
(Figure
2.
A).
Unlike
the
BALB/c
macrophages,
the
test
groups
of
C57BL/6
derived
macrophages
slightly
expressed
all
mentioned
genes
except
mBD6
more
than
their
controls,
but
there
wasn’t
observed
any
significant
differences
between
them
(Figure
2.
B).
Inter
assay
analysis
showed
that
the
test
groups
of
BALB/c
derived
macrophages
significantly
expressed
a
high
level
of
CRAMP
(3.2507±0.0499)
and
mBD1
(3.0362±0.0701)
compared
to
(0.9852±0.0267)
and
(1.2074±0.0418)
in
C57BL/6
derived
macrophages,
respectively
(Figure
2.
C
).
Cytokines
expression:
Cytokines
expression
was
assessed
using
real-time
PCR
method,
and
the
findings
analyzed
under
CT
method.
In
BALB/C
derived
macrophages,
the
test
groups
expressed
a
low
level
of
IL-12
(0.96
±
0.04)
and
a
significant
level
of
IL-10
(1.91±
0.02)
in
comparison
to
(1.005±0.0134)
and
(1.0016±0.0231)
for
their
controls,
respectively
(Figure
3.
A
).
Instead,
the
test
groups
of
C57BL/6
derived
macrophages
showed
significant
expression
levels
of
IL-12
(2.19±
0.05)
and
a
low
level
of
IL-10
(0.85
±
0.01)
versus
(1.0036±0.0347)
and
(1.0021±0.0260)
for
their
controls
(Figure
3.
B).
Protein
assay:
Based
on
AMPs
genes
expression,
the
test
groups
of
BALB/c
derived
macrophages
expressed
the
mRNA
levels
of
CRAMP
and
mBD1
more
than
those
in
C57BL/6.
Owing
to
higher
expression
of
CRAMP
compared
to
mBD1,
protein
assay
was
performed
only
for
CRAMP
7
days
post
infection.
Attention
to
data
detected
by
ELISA
method,
the
test
groups
of
BALB/c
derived
macrophages
released
a
high
level
of
CRAMP
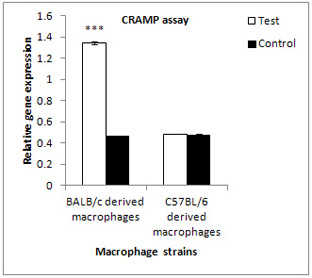
(1.3447±0.010497)
in
comparison
to
their
controls
(0.4706±0.002537)
and
to
(0.4862±0.0021)
and
(0.4803±0.0022)
for
the
other
strain
according
to
the
absorbance
of
optical
density
assessment
(Figure
4).
Click
here
for
Figures
1
A
&
B
Click
here
for
Figures
2
A,
B
&
C
Click
here
for
Figures
3
A
&
B
Figure
4
| DISCUSSION
AND
CONCLUSION
|
Leishmaniasis
is
a
public
health
problem
in
many
countries
(Stefaniak
et
al.,
2002)
and
there
are
an
estimated
700,000–1
million
new
cases
each
year
(WHO.
Fact
sheet.
April
2017).
It
takes
a
huge
economic
burden
annually.
L.
major
infection
is
an
appropriate
model
to
determine
the
necessity
of
immune
responses
to
infection
outcome.
It
has
been
proven
that
the
increase
of
some
immune
effectors,
such
as
IL-12,
cause
naive
lymphocytes
differentiate
to
Th2,
which
can
produce
IFN
cytokine
(Park
et
al.,
2000).
This
cytokine
plays
a
very
important
role
in
activating
of
macrophage
cells.
IFN-activated
macrophages
can
destroy
the
intracellular
parasites
through
a
variety
of
well
known
mechanisms
and
induce
resistance
in
C57BL/6
mouse
strain
(Assreuy
et
al.,
1994).
Instead
the
polarization
of
Th2
can
ultimately
predispose
BALB/c
mice
to
infection
(Chatelain
et
al.,
1992).
Similarly
to
in
vivo
model,
BALB/c
and
C57BL/6
derived
macrophages
represent
different
responses,
when
they
are
challenged
with
L.
major
parasites
(Rabhi
et
al.,
2013).
It
is
possible
for
genetically
different
cell
types
to
exhibit
an
unlike
response
to
the
same
pathogen
like
L.
major.
There
is
a
number
of
infections
referred
to
as
AMPs
imbalance,
in
which
they
influence
susceptibility
or
resistance
to
infections
(Rivas-Santiago
et
al.,
2009).
Surprisingly,
these
peptides
classified
in
the
innate
immunity
of
living
organisms,
can
kill
or
inhibit
pathogens
in
each
category
(Lehrer
and
Ganz,
2002a,
Lehrer
and
Ganz,
2002b,
Zasloff,
2002,
Bardan
et
al.,
2004,
Cavalcante
et
al.,
2017,
Kao
et
al.,
2016,
Mello
et
al.,
2017,
Vieira-Girao
et
al.,
2017).
They
can
be
used
as
new
drugs
or
applied
as
vaccine
and
resistance
to
them
is
rare
(Dabirian
et
al.,
2013,
Diamond,
2001,
Hancock
and
Sahl,
2006).
The
present
study
aimed
to
show
if
these
peptides
can
affect
susceptibility
or
resistance
to
L.
major
infection.
We
designed
an
in
vitro
model
for
studying
of
AMPs
in
parasitic
infection
for
the
first
time.
The
increased
level
of
infection
severity
in
BALB/c
derived
macrophages
indicates
that
this
type
is
more
sensitive
to
leishmania
infection
(Fig1.A-B).
According
to
Sunderkotter
et
al.
(Sunderkötter
et
al.,
1993),
C57BL/6
derived
macrophages
infected
by
L.
major
parasites
mature
faster,
which
results
in
the
reduction
of
their
infection
severity
and
susceptibility.
The
results
derived
from
this
survey
revealed
that
BALB/c
derived
macrophages
use
AMPs
especially
CRAMP
and
mBD1more
to
reduce
clinical
symptoms
(Figure
2.
A).
It
seems
that
their
susceptibility
to
infection
is
the
immense
criteria
for
the
increase
of
AMPs.
There
are
less
in
vitro
documented
studies
in
this
background
in
the
parasitic
field.
In
a
described
study,
human
macrophages
type
expressed
a
significant
up-regulation
of
CRAMP
between
the
macrophages
types
(Bank,
2012).
Other
research
has
been
generally
focused
on
in
vivo
models.
Radzishevsky
et
al.
(Radzishevsky
et
al.,
2005)
showed
that
CRAMP
knock-out
gene
mice
represent
a
severe
infectivity
rate
of
L.
amozonensis
infection
in
their
tissues
than
wild
type.
Another
aim
of
this
survey
was
the
study
of
cytokine
profiles.
Based
on
the
findings
related
to
cytokine
profiles,
BALB/c
derived
macrophages
expressed
a
significant
up-regulation
of
IL-10
and
a
low
level
of
IL-12,
while
the
other
type
showed
completely
reverse
reaction
(Figure
3.
A-B).
Data
from
a
previous
study
demonstrated
that
human
derived
macrophages
type
1
with
less
sensitivity
to
L.
major
infection
expressed
IL-12
more
against
other
types
(Bank,
2012).
Finally,
the
information
contained
in
mRNA
molecule
must
be
converted
to
the
synthesis
of
a
new
protein.
Due
to
higher
expression,
the
newly
synthesized
peptide
of
CRAMP
was
more
measured
for
BALB/c
derived
macrophages
than
the
other
type
(Figure
4).
Taken
together,
AMPs
consists
of
a
defense
barrier
against
L.
major
infection
especially
in
susceptible
macrophages,
but
cannot
create
an
absolute
protection
following
L.
major
infection.
Acknowledgment
We
are
grateful
from
Kerman
medical
university
owing
to
finance
supportive
burden
of
this
project
and
appreciatively
the
leishmaniasis
research
center
due
to
use
of
experience
and
their
requirements.
.Alvar,
J.,
Velez,
I.
D.,
Bern,
C.,
Herrero,
M.,
Desjeux,
P.,
Cano,
J.,
Jannin,
J.,
Den
Boer,
M.
&
Team,
W.
L.
C.
2012.
Leishmaniasis
worldwide
and
global
estimates
of
its
incidence.
PLoS
One,
7,
e35671.
Ashford,
R.
1997.
The
leishmaniases
as
model
zoonoses.
Annals
of
tropical
medicine
and
parasitology,
91,
693-702.
Assreuy,
J.,
Cunha,
F.
Q.,
Epperlein,
M.,
NoronhaDutra,
A.,
O’donnell,
C.
A.,
Liew,
F.
Y.
&
MoncadA,
S.
1994.
Production
of
nitric
oxide
and
superoxide
by
activated
macrophages
and
killing
of
Leishmania
major.
European
journal
of
immunology,
24,
672-676.
Azizi,
M.
H.,
Bahadori,
M.,
Dabiri,
S.,
Shamsi
Meymandi,
S.
&
Azizi,
F.
2016.
A
History
of
Leishmaniasis
in
Iran
from
19th
Century
Onward.
Arch
Iran
Med,
19,
153-62.
Bank,
E.
2012.
Leishmania
major
parasites
and
their
interaction
with
human
macrophages.
Universitätsbibliothek
Mainz.
Bardan,
A.,
Nizet,
V.
&
Gallo,
R.
L.
2004.
Antimicrobial
peptides
and
the
skin.
Expert
opinion
on
biological
therapy,
4,
543-549.
Cavalcante,
C.
S.,
Falcao,
C.
B.,
Fontenelle,
R.
O.,
Andreu,
D.
&
Radis-Baptista,
G.
2017.
Anti-fungal
activity
of
Ctn[15-34],
the
C-terminal
peptide
fragment
of
crotalicidin,
a
rattlesnake
venom
gland
cathelicidin.
J
Antibiot,
70,
231-237.
Chatelain,
R.,
Varkila,
K.
&
Coffman,
R.
L.
1992.
IL-4
induces
a
Th2
response
in
Leishmania
major-infected
mice.
The
Journal
of
Immunology,
148,
1182-1187.
Chromek,
M.,
Arvidsson,
I.
&
Karpman,
D.
2012.
The
antimicrobial
peptide
cathelicidin
protects
mice
from
Escherichia
coli
O157:H7-mediated
disease.
PLoS
One,
7,
15.
Dabirian,
S.,
Taslimi,
Y.,
Zahedifard,
F.,
Gholami,
E.,
Doustdari,
F.,
Motamedirad,
M.,
Khatami,
S.,
Azadmanesh,
K.,
Nylen,
S.
&
Rafati,
S.
2013.
Human
neutrophil
peptide-1
(HNP-1):
a
new
anti-leishmanial
drug
candidate.
PLoS
Negl
Trop
Dis,
7.
Deng,
Y.
Y.,
Shamoon,
M.,
He,
Y.,
Bhatia,
M.
&
Sun,
J.
2016.
Cathelicidin-related
antimicrobial
peptide
modulates
the
severity
of
acute
pancreatitis
in
mice.
Mol
Med
Rep,
13,
3881-5.
Deng,
Y.
Y.,
Shamoon,
M.,
He,
Y.,
Bhatia,
M.
&
Sun,
J.
2016.
Cathelicidin-related
antimicrobial
peptide
modulates
the
severity
of
acute
pancreatitis
in
mice.
Mol
Med
Rep,
13,
3881-5.
Desjeux,
P.
1996.
Leishmaniasis:
public
health
aspects
and
control.
Clinics
in
dermatology,
14,
417-423.
Diamond,
G.
2001.
Natures
antibiotics:
the
potential
of
antimicrobial
peptides
as
new
drugs.
Biologist
(London,
England),
48,
209.
Dorschner,
R.
A.,
Lin,
K.
H.,
Murakami,
M.
&
Gallo,
R.
L.
2003.
Neonatal
skin
in
mice
and
humans
expresses
increased
levels
of
antimicrobial
peptides:
innate
immunity
during
development
of
the
adaptive
response.
Pediatric
research,
53,
566-572.
Dostálová,
A.
&
Volf,
P.
2012.
Leishmania
development
in
sand
flies:
parasite-vector
interactions
overview.
Parasites
&
vectors,
5,
1.
Dubos,
R.
J.
1939.
Studies
on
a
bactericidal
agent
extracted
from
a
soil
bacillus:
I.
Preparation
of
the
agent.
Its
activity
in
vitro.
The
Journal
of
experimental
medicine,
70,
1.
Faber,
W.
R.,
Oskam,
L.,
Van
Gool,
T.,
Kroong,
N.
C.,
Knegt-Junk,
K.
J.,
Hofwegenf,
H.,
Van
Der
Wal,
A.
C.
&
Kager,
P.
A.
2003.
Value
of
diagnostic
techniques
for
cutaneous
leishmaniasis.
Journal
of
the
American
Academy
of
Dermatology,
49,
70-74.
Ganz,
T.
2003.
Defensins:
antimicrobial
peptides
of
innate
immunity.
Nature
Reviews
Immunology,
3,
710-720.
Hancock,
R.
E.
&
Sahl,
H.-G.
2006.
Antimicrobial
and
host-defense
peptides
as
new
anti-infective
therapeutic
strategies.
Nature
biotechnology,
24,
1551-1557.
HANDMAN,
E.
&
BULLEN,
D.
V.
2002.
Interaction
of
Leishmania
with
the
host
macrophage.
Trends
in
parasitology,
18,
332-334.
Hejazi,
S.,
Hoseini,
S.,
Javanmard,
S.,
Zarkesh,
S.
&
Khamesipour,
A.
2012.
Interleukin-10
and
Transforming
Growth
Factor-beta
in
Early
and
Late
Lesions
of
Patients
with
Leishmania
major
Induced
Cutaneous
Leishmaniasis.
Iran
J
Parasitol,
7,
16-23.
Hemshekhar,
M.,
Anaparti,
V.
&
Mookherjee,
N.
2016.
Functions
of
Cationic
Host
Defense
Peptides
in
Immunity.
Pharmaceuticals,
9.
Herwaldt,
B.
L.
1999.
Leishmaniasis.
The
Lancet,
354,
1191-1199.
KAO,
C.,
LIN,
X.,
YI,
G.,
ZHANG,
Y.,
ROWE-MAGNUS,
D.
A.
&
BUSH,
K.
2016.
Cathelicidin
Antimicrobial
Peptides
with
Reduced
Activation
of
Toll-Like
Receptor
Signaling
Have
Potent
Bactericidal
Activity
against
Colistin-Resistant
Bacteria.
MBio,
7,
01418-16.
Kovach,
M.
A.,
Ballinger,
M.
N.,
Newstead,
M.
W.,
Zeng,
X.,
Bhan,
U.,
Yu,
F.
S.,
Moore,
B.
B.,
Gallo,
R.
L.
&
Standiford,
T.
J.
2012.
Cathelicidin-related
antimicrobial
peptide
is
required
for
effective
lung
mucosal
immunity
in
Gram-negative
bacterial
pneumonia.
J
Immunol,
189,
304-11.
Lazarski,
C.
A.,
Ford,
J.,
Katzman,
S.
D.,
Rosenberg,
A.
F.
&
Fowell,
D.
J.
2013.
IL-4
attenuates
Th1-associated
chemokine
expression
and
Th1
trafficking
to
inflamed
tissues
and
limits
pathogen
clearance.
PLoS
One,
8.
Le
Blancq,
S.,
Schnur,
L.
&
Peters,
W.
1986.
Leishmania
in
the
Old
World:
1.
The
geographical
and
hostal
distribution
of
L.
major
zymodemes.
Transactions
of
the
Royal
Society
of
Tropical
Medicine
and
Hygiene,
80,
99-112.
Lehrer,
R.
I.
&
Ganz,
T.
2002a.
Cathelicidins:
a
family
of
endogenous
antimicrobial
peptides.
Current
opinion
in
hematology,
9,
18-22.
Lehrer,
R.
I.
&
GANZ,
T.
2002b.
Defensins
of
vertebrate
animals.
Current
opinion
in
immunology,
14,
96-102.
Mello,
C.
P.,
Lima,
D.
B.,
Menezes,
R.
R.,
Bandeira,
I.
C.,
Tessarolo,
L.
D.,
Sampaio,
T.
L.,
Falcao,
C.
B.,
Radis-Baptista,
G.
&
Martins,
A.
M.
2017.
Evaluation
of
the
antichagasic
activity
of
batroxicidin,
a
cathelicidin-related
antimicrobial
peptide
found
in
Bothrops
atrox
venom
gland.
Toxicon,
130,
56-62.
Nizet,
V.
&
Gallo,
R.
L.
2003.
Cathelicidins
and
innate
defense
against
invasive
bacterial
infection.
Scandinavian
journal
of
infectious
diseases,
35,
670-676.
Park,
A.
Y.,
Hondowicz,
B.
D.
&
Scott,
P.
2000.
IL-12
is
required
to
maintain
a
Th1
response
during
Leishmania
major
infection.
The
Journal
of
Immunology,
165,
896-902.
Rabhi,
I.,
Rabhi,
S.,
Ben-Othman,
R.,
Aniba,
M.
R.,
Trentin,
B.,
Piquemal,
D.,
Regnault,
B.
&
Guizani-Tabbane,
L.
2013.
Comparative
analysis
of
resistant
and
susceptible
macrophage
gene
expression
response
to
Leishmania
major
parasite.
BMC
Genomics,
14,
1471-2164.
Radzishevsky,
I.
S.,
Rotem,
S.,
Zaknoon,
F.,
Gaidukov,
L.,
Dagan,
A.
&
Mor,
A.
2005.
Effects
of
acyl
versus
aminoacyl
conjugation
on
the
properties
of
antimicrobial
peptides.
Antimicrobial
agents
and
chemotherapy,
49,
2412-2420.
Ray,
A.
&
Dittel,
B.
N.
2010.
Isolation
of
mouse
peritoneal
cavity
cells.
JoVE
(Journal
of
Visualized
Experiments),
e1488-e1488.
Rivas-Santiago,
B.,
Serrano,
C.
J.
&
Enciso-Moreno,
J.
A.
2009.
Susceptibility
to
infectious
diseases
based
on
antimicrobial
peptide
production.
Infection
and
immunity,
77,
4690-4695.
Stefaniak,
J.,
Paul,
M.,
Kacprzak,
E.
&
Skoryna-Karcz,
B.
2002.
[Visceral
leishmaniasis].
Przeglad
epidemiologiczny,
57,
341-348.
Sunderkötter,
C.,
Kunz,
M.,
Steinbrink,
K.,
Meinardus-Hager,
G.,
Goebeler,
M.,
Bildau,
H.
&
Sorg,
C.
1993.
Resistance
of
mice
to
experimental
leishmaniasis
is
associated
with
more
rapid
appearance
of
mature
macrophages
in
vitro
and
in
vivo.
The
Journal
of
Immunology,
151,
4891-4901.
VegaLópez,
F.
2012.
Leishmaniasis:
old
world.
Imported
Skin
Diseases,
189.
Vieira-Girao,
P.
R.,
Falcao,
C.
B.,
Rocha,
I.
R.,
Lucena,
H.
M.,
Costa,
F.
H.
&
Radis-Baptista,
G.
2017.
Antiviral
Activity
of
Ctn[15-34],
A
Cathelicidin-Derived
Eicosapeptide,
Against
Infectious
Myonecrosis
Virus
in
Litopenaeus
vannamei
Primary
Hemocyte
Cultures.
Food
Environ
Virol,
16,
017-9285.
WHO.
Fact
sheet
Updated
April
2017.
http://www.who.int/mediacentre/factsheets/fs375/en/
accessed
October16,
2017
Zasloff,
M.
2002.
Antimicrobial
peptides
in
health
and
disease.
New
England
Journal
of
Medicine,
347,
1199-1199.
Zhao,
X.,
Wu,
H.,
Lu,
H.,
Li,
G.
&
Huang,
Q.
2013.
LAMP:
a
database
linking
antimicrobial
peptides.
PLoS
One,
8,
e66557.
|

